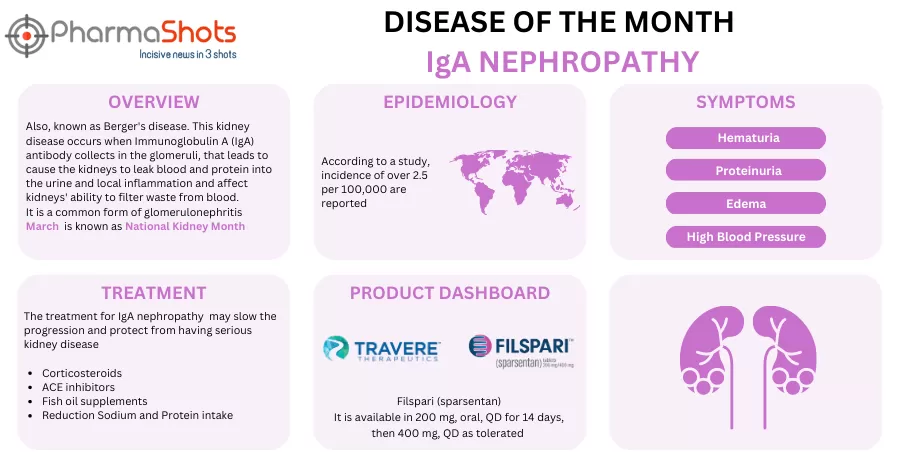
Travere Therapeutics Report Results for Filspari in 2 P-III Trial for the Treatment of IgA Nephropathy (IgAN) and Focal Segmental Glomerulosclerosis (FSGS)
Shots:
- The 2 P-III studies (PROTECT) & (DUPLEX) evaluated Filspari (Sparsentan, 400mg) vs irbesartan (300mg) in IgAN (n=404) & FSGS patients (n=370), the results for which were presented at the LANCET & NEJM respectively. Both studies fell short of the US FDA's expectations on total eGFR slopes, even after obtaining accelerated approval
- The (PROTECT) trial depicted a slow kidney function decline (-2.7 chronic eGFR slope &-2.9 ml/min/1.73m2/year total eGFR slope), consistent treatment effects across baseline eGFR (-5.8 vs -9.5 ml/min/1.73m2) & proteinuria (-43% vs -4%) & higher rates of complete remission (31% vs 11%)
- The (DUPLEX) trial showed proteinuria reduction (50% vs 32%), higher rates of proteinuria remission (18.5% vs 7.5%) & lower rates of end-stage kidney disease
Ref: Travere Therapeutics | Image: Travere Therapeutics
Related Posts:- Travere Therapeutics’ Filspari (sparsentan) Receives the US FDA’s Approval for the Reduction of Proteinuria in IgA Nephropathy
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release
Tags

Shivani was a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She was covering news related to Product approvals, clinical trial results, and updates. We can be contacted at connect@pharmashots.com.