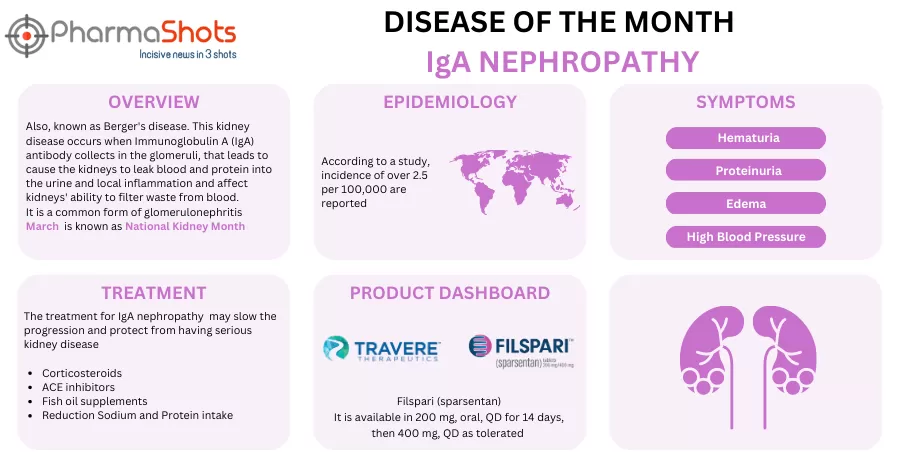
Travere Therapeutics’ Filspari (sparsentan) Receives the US FDA’s Approval for the Reduction of Proteinuria in IgA Nephropathy
Shots:
- The US FDA has granted accelerated approval to Filspari to reduce proteinuria in adults with primary IgAN who are at risk of rapid disease progression. Filspari is expected to be available in Feb 2023
- The approval was based on an ongoing (PROTECT) study evaluating sparsentan (400mg) vs irbesartan (300mg) in 404 patients aged ≥18yrs. which showed a significant improvement in proteinuria & the therapy was well tolerated with a consistent safety profile across all clinical trials
- The 2yr. results from the confirmatory EPs analysis are expected in Q4’23 & will support traditional approval of Filspari. The company has launched a patient support program Travere TotalCare incl. service, assistance & resources for eligible patients
Ref: Globenewswire | Image: Travere Therapeutics
Related Posts:- Travere Therapeutics Reports US FDA Acceptance of NDA and Priority Review for Sparsentan to Treat IgA Nephropathy
Click here to read the full press release
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.