Insights+: EMA Marketing Authorization of New Drugs in January 2023
Shots:
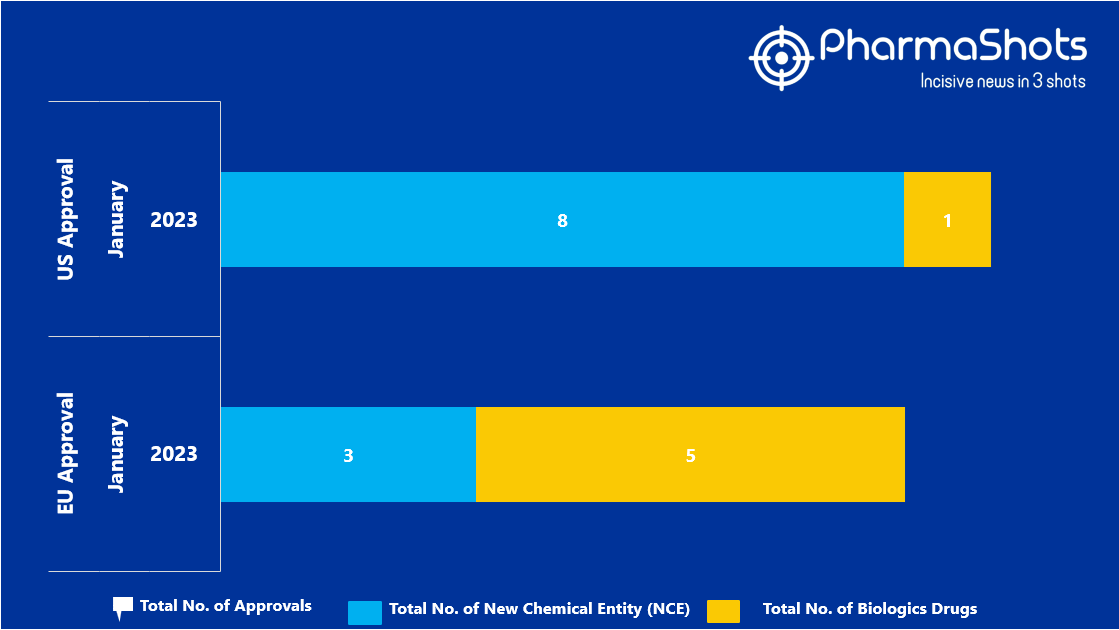
- The EMA approved 3 New Chemical Entity (NCE) and 5 Biologic Drugs in January 2023, leading to treatments for patients and advances in the healthcare industry
- In January 2023, the major highlights drugs were Xofluza’s Approval for influenza, Dupixent for eosinophilic esophagitis
- PharmaShots has compiled a list of a total of 8 new drugs approved by the EMA in January 2023
Trodelvy
Active ingredient: sacituzumab govitecan-hziy Approved: January 03, 2023
Company: Gilead Disease: Breast Cancer
- The EMA has validated a Type II variation MAA for Trodelvy to treat adult patients with unresectable or metastatic HR+, HER2- (IHC 0, IHC 1+ or IHC 2+/ISH–) breast cancer who have received endocrine-based therapy & 2 additional systemic therapies in the metastatic setting
- The MAA was based on the P-III study (TROPiCS-02) evaluating Trodelvy vs CT (eribulin, capecitabine, gemcitabine, or vinorelbine) in a ratio (1:1) in 543 patients. The trial met its 1EPs of PFS & 2EPs of OS. The PFS data were published in the Journal of Clinical Oncology & OS data were presented at ESMO 2022
- The safety profile was consistent with prior studies with no new safety signals. The US FDA has accepted the sBLA of Trodelvy for priority review in Oct 2022 & the PDUFA date is Feb 2023
Xofluza
Active ingredient: baloxavir marboxil Approved: January 12, 2023
Company: Roche Disease: Influenza
- Xofluza has been approved by the EC for use in children aged ≥1yr. for uncomplicated influenza & post-exposure prophylaxis of influenza
- The approval was based on the P-III study (miniSTONE-2) & (BLOCKSTONE) evaluating single-dose of Xofluza. The study (miniSTONE-2) met its 1EPs of safety & showed a reduction in the length of time that influenza was released from the body by ≥2 days vs oseltamivir (median time of 24.2 vs 75.8hrs.), was well tolerated with no new safety signals
- In the (BLOCKSTONE) study, significant prophylactic effects were observed after a single oral dose by lowering the risk of individuals after exposure to an infected household member (1.9% in Xofluza vs 13.6% in PBO). Xofluza has approved in 70+ countries for influenza types A & B
AstraZeneca’s Tezspire (tezepelumab) Receives the EU Approval for the Treatment of Severe Asthma
Tezspire
Active ingredient: tezepelumab Approved: January 13, 2023
Company: AstraZeneca Disease: Asthma
- The EMA’s CHMP issued a positive opinion on Tezspire (tezepelumab) for self-administration in a pre-filled, single-use pen for patients aged ≥12yrs. with sev. asthma
- The approval was based on the (PATHFINDER) program incl. results from the P-I trial (PATH-BRIDGE) & P-III trial (PATH-HOME) evaluating Tezspire (210mg). In the (PATH-HOME) trial, 92% of healthcare providers, patients, and caregivers were able to successfully administer Tezspire in the clinic & home along with an improvement in asthma control & safety profile was consistent with prior trials
- The US FDA’s decision on self-administration and the new pre-filled pen is expected in H1’23. The therapy was approved in the US, EU, Japan & other countries for the treatment of sev. asthma
Enhertu
Active ingredient: trastuzumab deruxtecan Approved: January 26, 2023
Company: Daiichi Sankyo and AstraZeneca Disease: Breast Cancer
- The EC has approved Enhertu (HER2-directed ADC) as monotx. for unresectable or metastatic HER2 low (IHC 1+ or IHC 2+/ISH-) breast cancer who received prior CT in the metastatic setting or developed disease recurrence during or within 6mos. of completing adjuvant CT
- The approval was based on the P-III trial (DESTINY-Breast04) evaluating Enhertu (5.4mg/kg) vs CT in a ratio (2:1) in 557 patients at multiple sites in Asia, EU & North America, showed a 50% reduction in risk of disease progression or death, m-PFS (9.9 vs 5.1mos.), 36% reduction in risk of death with m-OS (23.4 vs 16.8mos.)
- The safety profile was consistent with prior trials with no new safety concerns. Enhertu (6.4mg/kg) was approved in 30+ countries for HER2+ gastric or GEJ adenocarcinoma
Sotyktu
Active ingredient: deucravacitinib Approved: January 27, 2023
Company: BMS Disease: Plaque Psoriasis
- The opinion was based on the P-III trials (POETYK PSO-1 & 2) results evaluating the safety & efficacy of Sotyktu (6mg, qd) vs PBO and Otezla (30mg, BID) in 666 & 1020 patients along with an additional 2yr. results from the (POETYK PSO) long-term extension trial
- The results showed significant & clinical improvements in skin clearance, symptom burden & QoL. The therapy was well-tolerated with a low rate of discontinuation due to AEs & both trials results were published in the JAAD
- Sotyktu was approved in the US for adults with mod. to sev. PsO in Sept 2022, and in Japan for PsO, GPP & erythrodermic psoriasis in Sept 2022. The therapy is currently under regulatory review by other health authorities globally
Pfizer Receives EMA’s CHMP Positive Opinion of Paxlovid for COVID-19
Paxlovid
Active ingredient: nirmatrelvir and ritonavir Approved: January 27, 2023
Company: Pfizer Disease: COVID-19
- The EMA’s CHMP issued a positive opinion recommending the conversion of cMA for Paxlovid (SARS-CoV-2 Mpro inhibitor) to full MA for adults with COVID-19 who do not require supplemental oxygen & are at increased risk of the disease becoming severe. The EC’s final decision is expected shortly
- The recommendation was based on the totality of efficacy, safety & quality data. Paxlovid’s advantages in assisting in lowering sev. COVID-19-related outcomes, such as hospitalization and death in high-risk patients continue to outweigh its potential risks
- Paxlovid was approved or authorized for conditional or emergency use in 70+ countries. It is generally administered at 300mg (two 150 mg tablets) of nirmatrelvir with one 100mg of ritonavir, BID for 5 days
Dupixent
Active ingredient: dupilumab Approved: January 27, 2023
Company: Regeneron Disease: Atopic Dermatitis
- The EMA’s CHMP has adopted a positive opinion recommending expanded approval for Dupixent to treat sev. AD. The EC’s final decision is expected in the coming months
- The opinion was based on the P-III trial results evaluating Dupixent (200/300mg, q4w) added to SoC low-potency TCS vs low-potency TCS alone in 162 children aged 6mos. to 5yrs.
- The trial met all 1EPs & 2EPs i.e., Dupixent + low-potency TCS showed improvement in skin clearance & reduced overall disease severity @16wks., reduction in itch & skin pain along with improved sleep quality & health-related QoL while the Long-term data showed a sustained improvement in the disease measures @~1yr. & the safety results were consistent with the known safety profile of Dupixent
Dupixent
Active ingredient: dupilumab Approved: January 30, 2023
Company: Regeneron and Sanofi Disease: Eosinophilic Esophagitis
- The EC has expanded the marketing authorization for Dupixent in adults & adolescents aged ≥12yrs. The EC’s decision was based on the 52wk. results from a P-III trial consisting of 3 parts i.e., parts A, B, C (n=42, 79, 188) evaluating Dupixent (300mg, qw) vs PBO for 24wks.
- The part A & B results showed ~10 times higher rate of histological remission (60% & 59% vs 5% & 6%); reduction in disease symptoms (69% & 64% vs 32% & 41%), clinical improvement (21.9 & 23.8-point vs 9.6 & 13.9-point)
- ≥7-fold reduction in abnormal endoscopic (-3.2 & -4.5 points vs -0.3 & -0.6 points), improvement in swallowing-related pain & health-related QoL & less frequent non-swallowing symptoms while long term efficacy in part C was similar to parts A & B results
Note: Sotyktu, Paxlovid & Dupixent received EMA’s CHMP Positive Opinion and EMA’s Validation of MAA for Trodelvy
Related Post: Insights+: EMA Marketing Authorization of New Drugs in December 2022





