Insights+: EMA Marketing Authorization of New Drugs in December 2022
Shots:
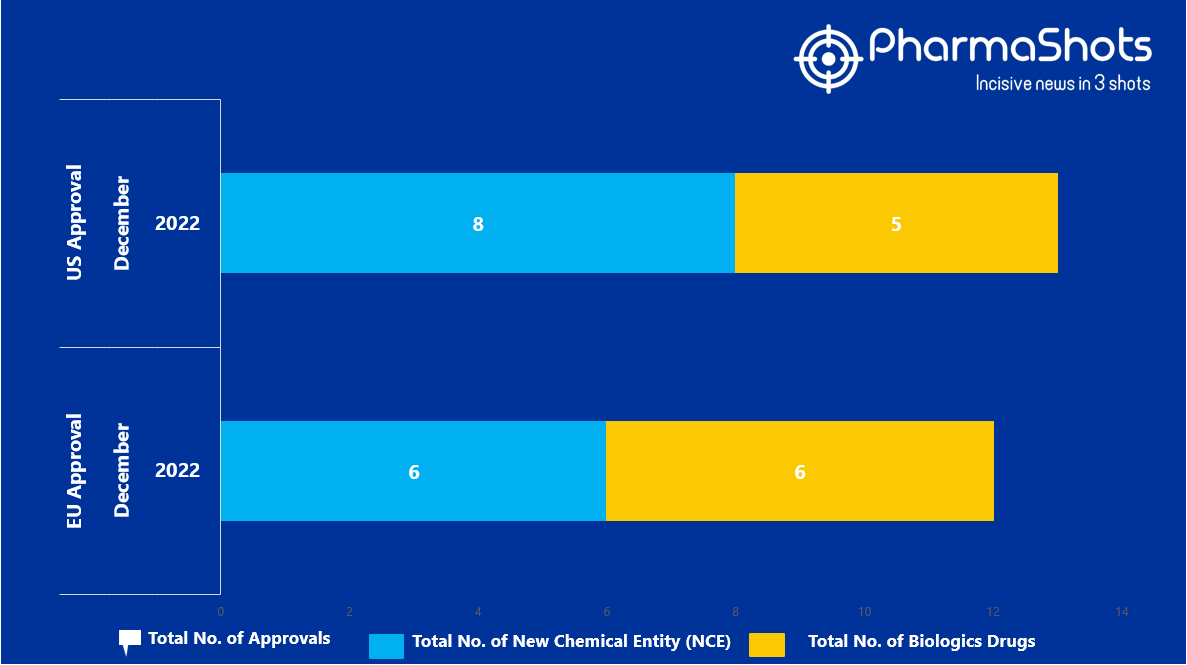
- The EMA approved 6 New Chemical Entity (NCE) and 6 Biologic Drugs in December 2022, leading to treatments for patients and advances in the healthcare industry
- In December 2022, the major highlights drugs were Pluvicto’s approval for progressive PSMA+ metastatic castration resistant prostate cancer, Dupixent for Prurigo Nodularis
- PharmaShots has compiled a list of a total of 12 new drugs approved by the EMA in December 2022
Qdenga
Active ingredient: dengue tetravalent vaccine Approved: December 08, 2022
Company: Takeda Disease: Dengue Disease
- The approval was based on the results from the 19 P-I/II/III trials incl. 4 & a half yrs. of follow-up data from P-III trial (TIDES) evaluating 2 doses of TAK-003 across Latin America & Asia
- The P-III (TIDES) trial met its 1EPs & 2EPs i.e., the vaccine showed an overall vaccine efficacy by preventing 80.2% of symptomatic dengue cases @12mos., 90.4% reduction in hospitalizations @18mos. In an exploratory analysis, 84% reduction in hospitalized dengue cases & 61% of symptomatic dengue cases @ 4.5yr. of follow-up, was well tolerated with no evidence of disease enhancement in vaccine recipients
- The vaccine was approved in Indonesia in individuals aged 6-45yr. while regulatory filings continue to progress in other dengue-endemic countries in Asia & Latin America
Pluvicto
Active ingredient: lutetium Lu 177 vipivotide tetraxetan Approved: December 13, 2022
Company: Novartis Disease: Prostate Cancer
- The approval was based on the P-III study (VISION) results evaluating the efficacy and safety of Pluvicto (7.4 GBq, IV, q6w for a maximum of 6 cycles) + BSoC vs BSoC in a ratio (2:1) in 831 patients with PSMA PET-scan positive mCRPC who have received AR pathway inhibition and taxane-based CT
- The result showed a 38% reduction in risk of death, a 60% reduction in risk of rPFS, ORR (30% vs 2%) in patients with evaluable disease at baseline
- In Oct 2022, the CHMP issued a positive opinion and is applicable to all 27 EU member states, Iceland, Norway, Northern Ireland & Liechtenstein. Pluvicto is also approved in the US and other countries incl. Great Britain and Canada for the same indication
Livmarli
Active ingredient: maralixibat Approved: December 13, 2022
Company: Mirum Disease: Alagille Syndrome
- The approval was based on the P-IIb (ICONIC) OLE study incl. 6yr. data of Livmarli vs PBO in patients with ALGS
- The (ICONIC) study showed a reduction in pruritus with a mean difference of -1.4 points, reductions in serum bile acids which were durably maintained over multiple yrs. of treatment, reductions in xanthoma severity, cholesterol & improvements in transplant-free survival & growth
- The product is expected to be available in Germany in early 2023 & is being studied in late-stage clinical studies in other rare cholestatic liver diseases & also received BTD for ALGS & PFIC type 2; ODD for ALGS, PFIC & biliary atresia. The therapy is currently under regulatory review in China, Taiwan, South Korea, and Great Britain
Dupixent
Active ingredient: dupilumab Approved: December 15, 2022
Company: Sanofi and Regeneron Disease: Prurigo Nodularis
- The approval was based on P-III trial (PRIME) & (PRIME2) evaluating Dupixent vs PBO with/out topical treatments in 151 & 160 patients with uncontrolled PN
- In both trials, 44% & 37% vs 16% & 22% experienced a reduction in itch @12wks. while an improvement was increased @24wks. where 60% & 58% vs 18% & 20% experienced a reduction in itch from baseline, clear or almost clear skin (48% & 45% vs 18% & 16%) @24wks., improved health-related QoL while reducing measures of skin pain & symptoms of anxiety/depression from baseline
- The safety results were consistent with the known safety profile of Dupixent in its approved indications. The product received an additional 1yr. marketing protection in the EU, following CHMP recommendation
Dupixent
Active ingredient: dupilumab Approved: December 16, 2022
Company: Regeneron Disease: Eosinophilic Esophagitis
- The EMA’s CHMP has adopted a positive opinion recommending approval of Dupixent for adults and adolescents with EoE. The EC’s decision is expected in the coming months
- The opinion was based on the 52wk. results from a P-III trial consisting of 3 parts (Part A in 81 patients), Part B (240 patients) & Part C evaluating Dupixent (300mg, qw) vs PBO for 24wks.
- The results showed an improvement in the ability to swallow as early as 4wks. as well as histological disease remission, improvements in abnormal endoscopic findings of the esophagus & cellular improvements @24wks. with outcomes maintained ~1yr. The safety results were consistent with the known safety profile of Dupixent in its approved indications
Spevigo
Active ingredient: spesolimab Approved: December 18, 2022
Company: Boehringer Ingelheim Disease: Generalized Pustular Psoriasis Flares
- The EC has granted conditional marketing authorization for spesolimab to treat adult patients with GPP flares
- The EC’s conditional approval was based on the P-II trial (EFFISAYIL 1) results of spesolimab vs PBO which showed that patients experienced a GPP flare @12wk., 54% vs 6% showed no visible pustules & AEs (66% vs 56%) after 1wk., infections were reported (17% vs 6%) & SAEs were reported in 6%
- Spesolimab is a novel selective Ab that blocks the activation of the interleukin-36 receptor & is already approved in the US & Japan for GPP. The therapy is also under investigation for the prevention of GPP flares & for other neutrophilic skin diseases
Hemgenix
Active ingredient: Etranacogene Dezaparvovec Approved: December 19, 2022
Company: uniQure Disease: Hemophilia B
- The EMA’s CHMP has adopted the positive opinion recommending CMA for etranacogene dezaparvovec in adult patients with hemophilia B
- The opinion was based on the P-III (HOPE-B) trial evaluating etranacogene dezaparvovec in 54 patients which showed a stable & durable increase in mean Factor IX (FIX) activity levels (with a mean FIX activity of 36.9%), 64% ABR reduction for all bleeds & 77% for all FIX-treated bleeds @6-18mos., 96% discontinued routine FIX prophylaxis with an overall 97% reduction in mean unadjusted annualized FIX consumption
- The 24mos, analysis showed a sustained & durable effect, was well-tolerated with no serious TRAEs with no correlation b/w patient AAV5 NAb levels at baseline & FIX activity
Fintepla
Active ingredient: fenfluramine Approved: December 19, 2022
Company: UCB Pharma Disease: Seizures
- The EMA’s CHMP positive opinion was based on the safety & efficacy data from the P-III trial in 263 patients aged 2-35yrs. The EC’s final decision is expected in Q1’23
- The results showed a greater reduction in the frequency of drop seizures at a dose of 0.7/mg/kg/day & additional results of fenfluramine in the OLE part of the study showed a sustained reduction in the frequency of multiple seizure types and was well tolerated at a median treatment duration of 364 days with no cases of valvular heart disease or pulmonary arterial hypertension
- Fenfluramine was approved in the US for seizures associated with Lennox- Gastaut syndrome & in the EU, US, and Japan for seizures associated with Dravet syndrome
Forxiga
Active ingredient: dapagliflozin Approved: December 19, 2022
Company: AstraZeneca Disease: Symptomatic Chronic Heart Failure
- The EMA’s CHMP positive opinion was based on the P-III (DELIVER) trial evaluating Forxiga vs PBO in 6263 patients with HF with LVEF ≥40% with/out T2D
- The results showed a 16.4% vs 19.5% reduction in the composite outcome of CV death or worsening of HF in patients with HFpEF & HFmrEF over a median follow-up of 2.3yrs. The treatment effect was consistent across the LVEF range without evidence of attenuation of effect by LVEF & the results were published in The NEJM
- In the pre-specified, patient-level, pooled analysis, Forxiga showed a 14% reduction in risk of CV death, death from any cause (10%) & total hHF (29%) over the median follow-up of 22mos. Forxiga was approved for HFrEF in 100+ countries globally incl. the US, the EU, China & Japan
Ebvallo
Active ingredient: tabelecleucel Approved: December 20, 2022
Company: Atara Biotherapeutics Disease: EBV+ PTLD
- The EC has granted marketing authorization for Ebvallo as a monotx. in adult and pediatric patients aged ≥2yrs. with r/r EBV+ PTLD who have received one prior therapy.
- The opinion was based on the P-III study (ALLELE) study evaluating the efficacy & safety of tabelecleucel in 66 patients with EBV + PTLD after the failure of rituximab & rituximab + CT (SOT cohort) or HCT after the failure of rituximab (HCT cohort). The results demonstrated a favorable risk-benefit profile
- The CHMP issued a positive opinion & applies to all 27 EU member states, Iceland, Norway & Liechtenstein. Under the licens agreement with Atara, Pierre Fabre will be responsible for all commercialization & distribution activities in the EU & other markets
Zynlonta
Active ingredient: loncastuximab tesirine Approved: December 21, 2022
Company: Sobi and ADC Therapeutics Disease: Diffuse Large B-Cell Lymphoma
- The EC has granted conditional marketing authorization to Zynlonta for the treatment of r/r DLBCL
- The approval was based on data from the P-II trial (LOTIS-2) evaluating Zynlonta in 145 adult patients with r/r DLBCL, following two or more prior lines of systemic therapy. The results showed an ORR of (48.3%) incl. CR rate of 24.1% and a PR rate of 24.1%, patients had a median time to response of 1.3mos. and m-DoR for the 70 responders was 10.3mos.
- The EC’s decision will be valid in all EU member states, Iceland, Norway & Liechtenstein. Sobi & ADC Therapeutics collaborated to develop and commercialize Zynlonta for use in haematology and other indications in the EU and most international markets
Lynparza
Active ingredient: olaparib Approved: December 21, 2022
Company: AstraZeneca and Merck Disease: Prostate Cancer
- The approval was based on the P-III trial (PROpel) results evaluating Lynparza vs PBO when given in addition to abiraterone, prednisone, or prednisolone in men with mCRPC who had not received prior CT or NHAs in the mCRPC setting
- The combination therapy showed a 34% reduction in risk of disease progression or death, m-rPFS (24.8 vs 16.6mos.). The planned sensitivity analysis was consistent with the investigator-based analysis with an m-rPFS of 27.6 vs 16.4mos.
- The interim results for 2EPs of OS did not reach statistical significance, the event rate (37.1% vs 43.1%), safety & tolerability were consistent with individual therapies, and AEs leading to treatment discontinuation (~16%). The therapy was approved in the US as monotx. for HRR gene-mutated mCRPC
Note: Spevigo & Zynlonta Received EC’s Conditional Marketing Authorization; Dupixent, Etranacogene Dezaparvovec, and Fintepla received EMA’s CHMP Positive Opinion
Related Post: Insights+: EMA Marketing Authorization of New Drugs in November 2022





