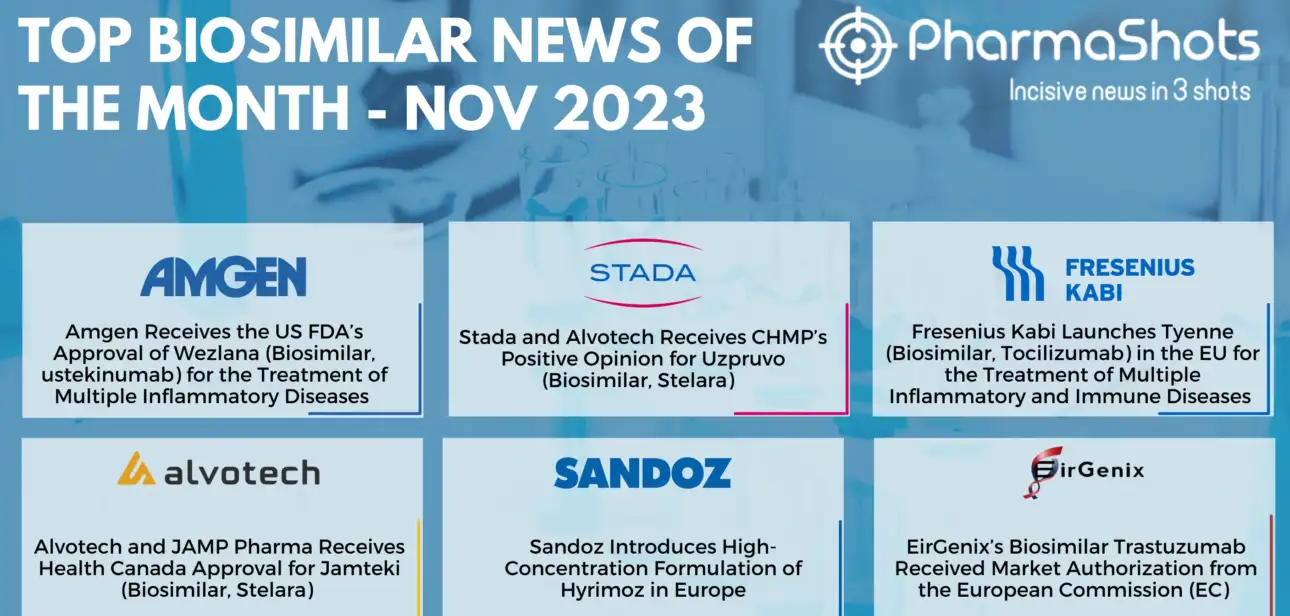
Shots:
Stay up to date with PharmaShots Biosimilars Report, a monthly digest designed to keep you familiarized with the recent developments in biologics
Biosimilars are cost-effective alternatives to branded drugs but are very much alike when it comes to safety and efficacy
Check out the developments in the Biosimilars with PharmaShots’ illuminating report. PharmaShots has…