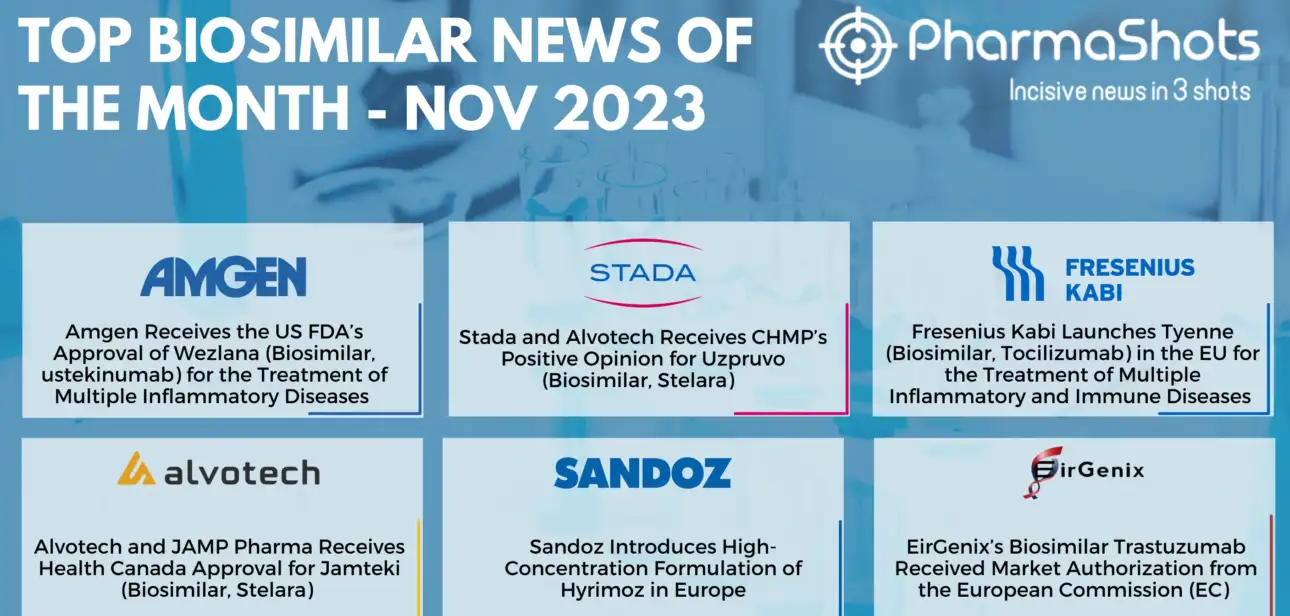
Shots:Biosimilars are developed to be highly similar versions of approved biologics in terms of safety, purity, and potency Biosimilars are expected to be a cost-effective alternative to the high-priced branded biologics, offering significant and much-needed cost savings to both payers and patients During November, Amgen received US FDA approval for Wezlana, biosimilar of ustekinumab &…