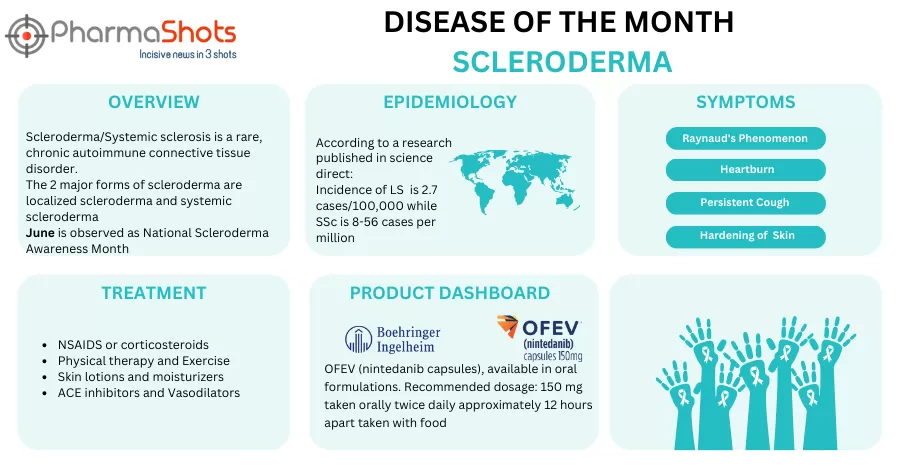
Shots: To keep our readers acquainted with several disease conditions, ongoing trials, and available treatment options, PharmaShots brings every month a detailed take on a particular disease after thorough research Continuing the series for the disease of the month, PharmaShots brings this month a summary of the disease, Scleroderma, a rare neurological disease that affects…