Active Ingredients: Adalimumab
Strength: 40 mg/0.8 ml
Dosage Form: Syringe, Vial
Mechanism of Action: TNF-alpha inhibitors
First Approval: US (Dec 31, 2002), EU (Aug 09, 2003)
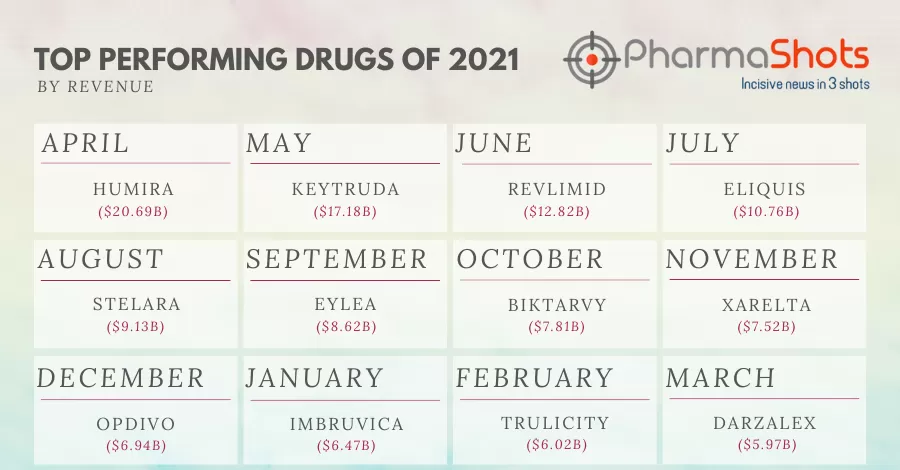
Revenue
Humira (adalimumab) has been the top-selling biologic drug product for the last few years – reaching nearly $21B in annual sales in 2021. Humira is one of the main drivers of AbbVie’s immunology…