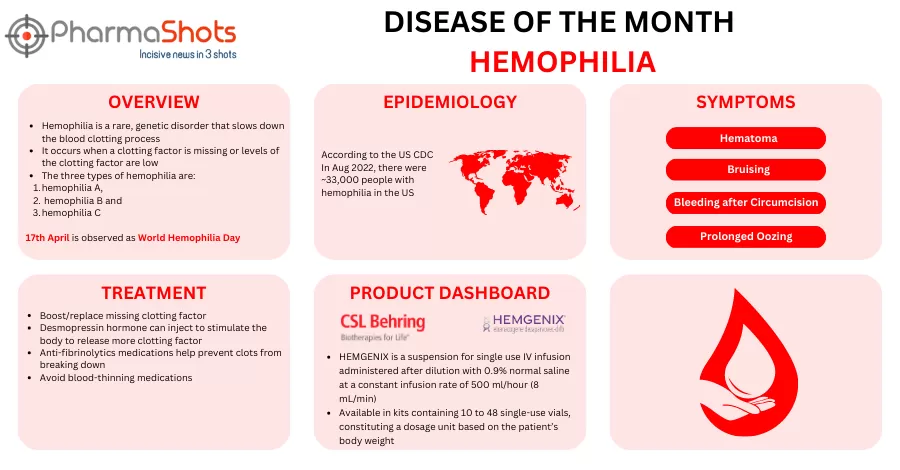
Shots:
The US FDA has accepted the BLA for Mim8 (denecimig; pre-filled, single-use pen) as routine prophylaxis to prevent or reduce bleeding episodes in adult & pediatric pts with hemophilia A (congenital FVIII deficiency), with or without inhibitors
BLA was supported by FRONTIER2 trial assessing Mim8 (QW/QM) in pts (≥12yrs), FRONTIER3 trial assessing it in…