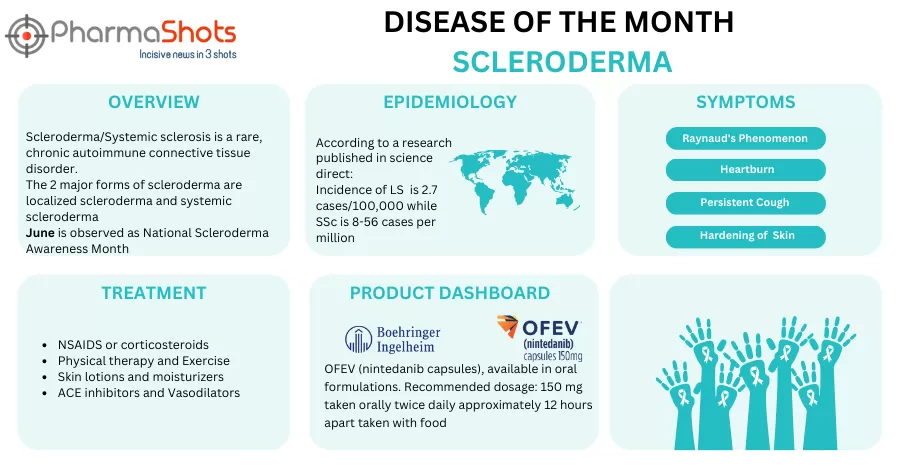
Shots:The US FDA approved 5 NDAs and 2 BLA in July 2023, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 76 novel products in 2023In July 2023, the major highlights drugs were Beyfortus (nirsevimab) approval for the prevention of RSV lower respiratory tract disease in…