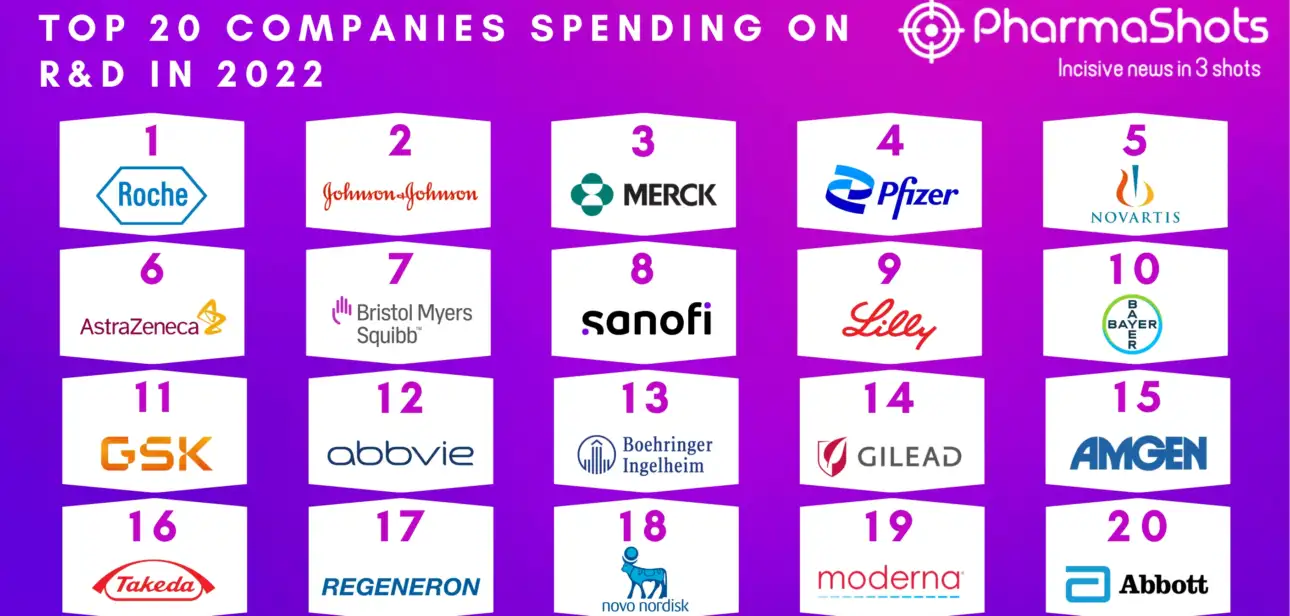
Shots: Witness the journey of the biopharma companies over 20 years with this informative and engaging report. The report highlights the development of the biopharma industry and the key factors influencing them In 2023, JNJ reclaims the topmost position in the list from Pfizer with a revenue of $85.16B, showcasing a sharp 10.30 percent vs…