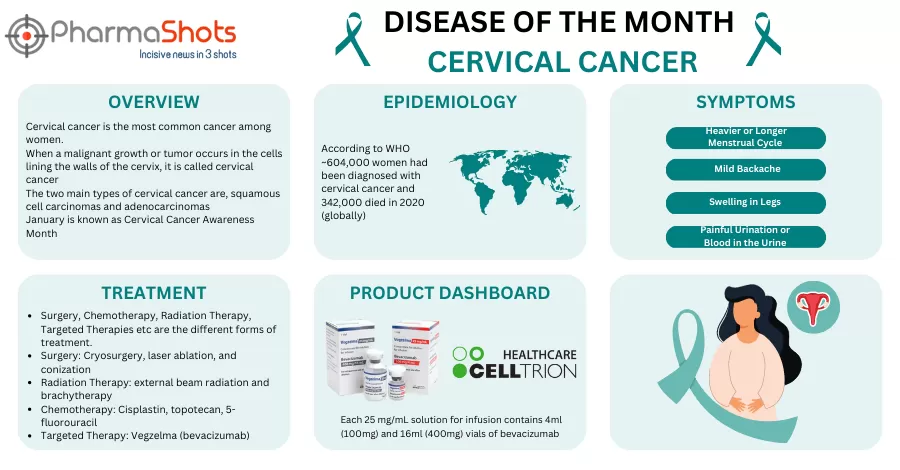
Shots:
The US FDA has approved Jobevne, a biosimilar version of Avastin (bevacizumab) for IV administration
Approval was based on extensive data showing Jobevne is similar to Avastin in PK, safety, efficacy, structure, & function across clinical & analytical studies
Jobevne (VEGF inhibitor) is marketed under the brand name Abevmy in the EU & Canada,…