
EMA Marketing Authorization of New Drugs in September 2024
Shots:
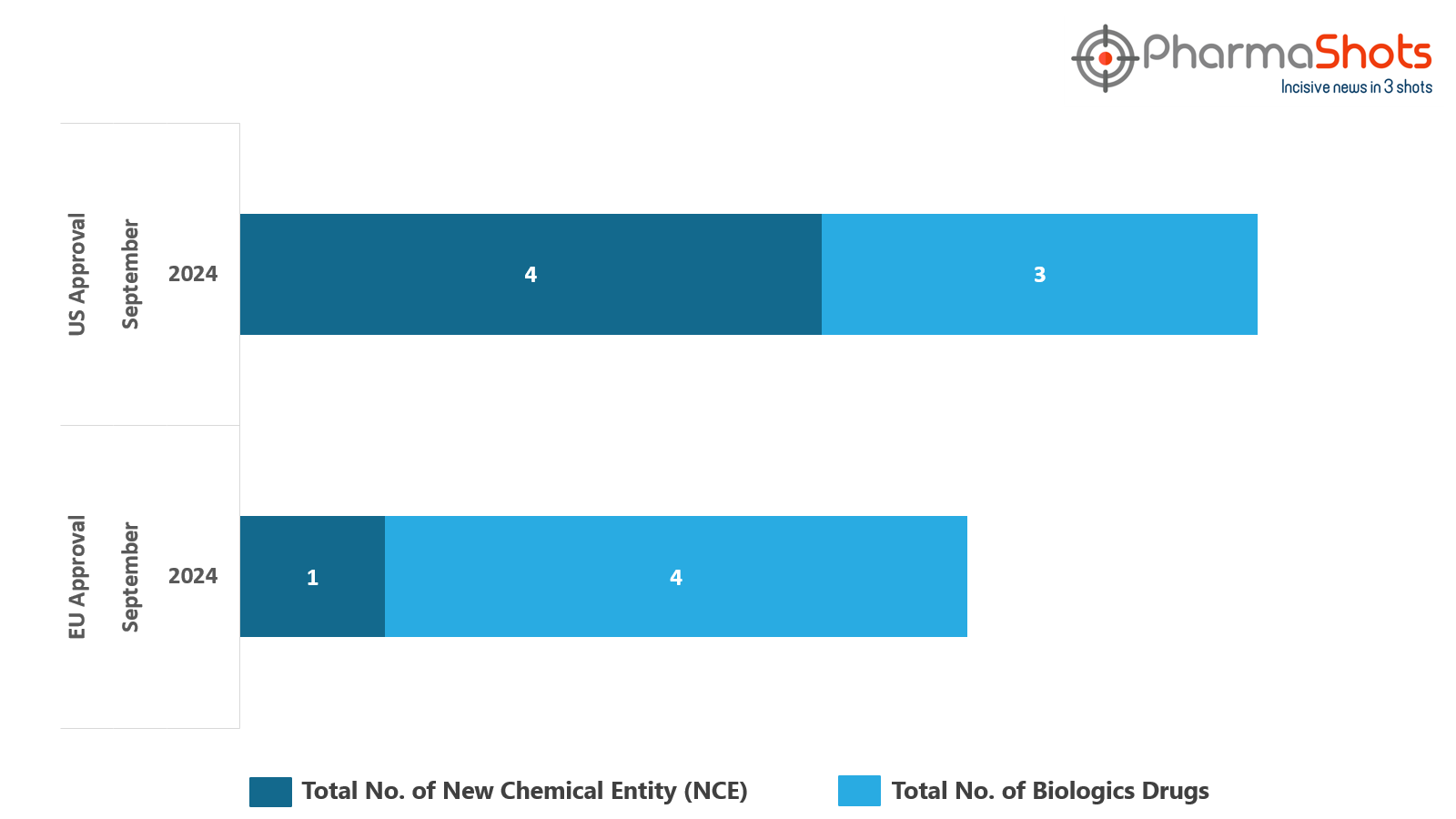
- The EMA’s CHMP has granted positive opinion to 4 Biologics and 1 New Chemical Entity in September 2024, leading to treatments for patients and advances in the healthcare industry
- The major highlighted drugs were AbbVie’s Elahere to treat Ovarian Cancer and Pfizer’s Hympavzi for Hemophilia A and B
- PharmaShots has compiled a list of 3 drugs that have been granted positive opinion by the EMA’s CHMP
Product Name: Elahere
Active ingredient: Mirvetuximab Soravtansine
Company: AbbVie
Date: Sep 19, 2024
Disease: Ovarian Cancer
Shots:
- The CHMP has granted positive opinion to Elahere for treating FRα+, Pt-resistant & high-grade serous epithelial ovarian, fallopian tube or primary peritoneal cancer in adults who have received 1 to 3 prior therapies. The decision is anticipated in Q4’24, with other reviews underway
- The opinion was based on P-III (MIRASOL) study assessing Elahere vs CT in 453 ovarian cancer patients who had received 1-3 lines of prior treatments
- Study showed improved OS with mOS of 16.46mos. vs 12.75mos., a 33% reduction in risk of death, improved PFS with a 35% reduction in risk of tumor progression or death with an mPFS of 5.62mos. vs 3.98mos. and ORR of 42.3% (incl. 12 CRs) vs 15.9% (with no CRs)
Product Name: Hetronifly
Active ingredient: Serplulimab
Company: Henlius and Intas
Date: Sep 19, 2024
Disease: Extensive-Stage Small Cell Lung Cancer
Shots:
- The positive opinion of Hetronifly (anti-PD-1 mAb) was supported by ASTRUM-005 study assessing it with CT vs PBO as a 1L treatment of ES-SCLC patients (n=585) in various regions. It is approved in China under the brand name Hansizhuang for the same
- Henlius partnered with Intas in 2023 to develop & commercialize Hetronifly across >50 countries in the EU & India. Intas’ subsidiary, Accord Healthcare, will commercialize the drug in the EU on approval
- In addition, the NMPA has accepted the application of Hetronifly as a 1L treatment of non-squamous NSCLC. It is further being assessed under P-III global study with CT & radiotherapy for LS-SCLC and under a bridging head-to-head US trial in comparison to atezolizumab (SoC) as a 1L treatment of ES-SCLC
3. Pfizer Reports the CHMP’s Positive Opinion of Hympavzi (Marstacimab) to Treat Hemophilia A and B
Product Name: Hympavzi
Active ingredient: Marstacimab
Company: Pfizer
Date: Sep 19, 2024
Disease: Hemophilia A and B
Shots:
- The CHMP has recommended Hympavzi (QW, SC) as a prophylactic treatment to prevent bleeding episodes in patients (≥12yrs.) with hemophilia A & B without FVIII & FIX inhibitors, respectively
- The opinion was based on data of 116 subjects (12-75yrs.) from a pivotal P-III (BASIS) study treated with marstacimab (300mg loading dose followed by 150mg then 300mg, QW) for over 12mos. active treatment period vs RP or OD IV treatments for 6mos. observational period. Results from ongoing inhibitor cohort are anticipated in Q3’25
- The EC’s decision is anticipated in the upcoming mos. and will be valid across the EU plus Iceland, Liechtenstein & Norway. It is also under the US FDA’s review (PDUFA: Q4’24)
Note: The following drugs have also been recommended for approval, however, no PR was available:
- Penbraya
- Theralugand
Related Post: Insights+: EMA Marketing Authorization of New Drugs in August 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














