
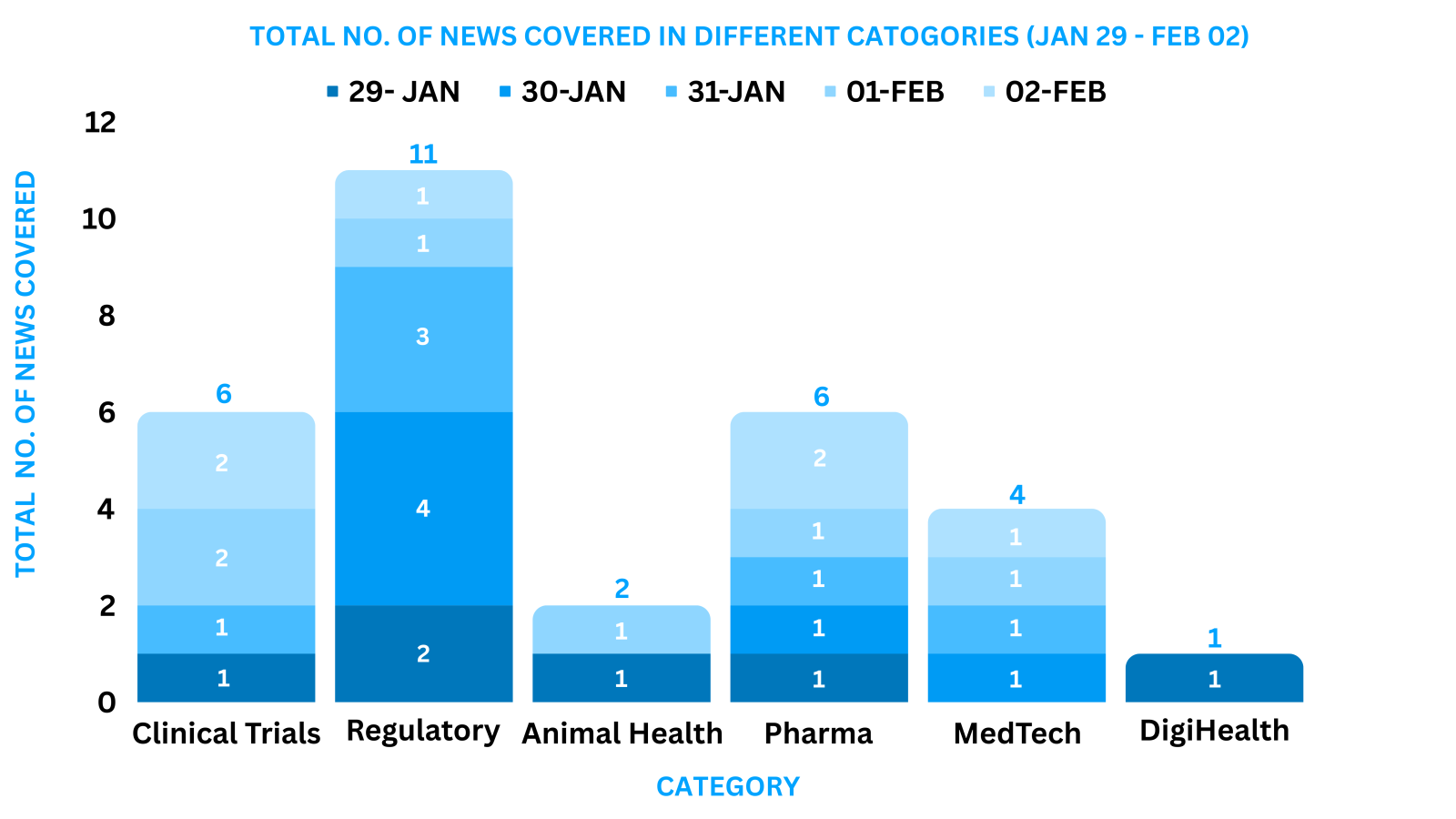
PharmaShots Weekly Snapshots (January 29 – February 02, 2024)
This week PharmaShots’ news was all about the updates on M&A, Pharma, Clinical Trials, Regulatory & MedTech. Check out our full report below:

The CHMP Grants BMS’ with Positive Opinion for Abecma (idecabtagene vicleucel) to Treat Multiple Myeloma
Read More: BMS
GSK’s Omjjara (momelotinib) Receives the European Commission Approval for the Treatment of Severe Anaemia
Read More: GSK
The Pharmacy and Poisons Board of Hong Kong Approves HUTCHMED’s Elunate (fruquintinib) for Metastatic Colorectal Cancer (CRC)
Read More HUTCHMED
The US FDA Approves Takeda’s GAMMAGARD LIQUID to Treat Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
Read More Takeda
The US FDA Accepts AstraZeneca and Daiichi Sankyo’s sBLA for Enhertu and Grants Priority Review for the Treatment of HER2-Expressing Solid Tumors
Read More AstraZeneca & Daiichi Sankyo
The US FDA Grants IND Clearance to Gracell Biotechnologies’s GC012F to Treat Multiple Myeloma
Read More Gracell Biotechnologies
Daiichi Sankyo Submits sNDA to the Japan MHLW for Ezharmia as a Treatment of Peripheral T-Cell Lymphoma (PTCL)
Read More Daiichi Sankyo
Astellas Submits sNDA for Padcev in Combination with Keytruda to the Japan MHLW for the Treatment of Advanced Bladder Cancer
Read More Astellas
The US FDA and Japan MHLW Accept BMS’ sBLA for Breyanzi to Treat Follicular Lymphoma (FL) and Mantle Cell Lymphoma (MCL)
Read More BMS
USGI Medical’s POSE2.0 Incisionless Weight Loss Procedure Receives US FDA’s IDE Approval for Primary Obesity
Read More USGI Medical
SeaStar Medical’s Selective Cytopheretic Device Technology Obtains Canadian Patent
Read More SeaStar Medical

Merck Highlights the P-III Trial Data for Keytruda (Pembrolizumab) as an Adjuvant Therapy for Urothelial Carcinoma
Read More: Merck
Vertex Highlights Results from Two P-III Analysis of VX-548 for the Treatment of Moderate-to-Severe Acute Pain
Read More Vertex
Genentech Presents Results from P-III (BALATON and COMINO) Studies of Vabysmo for Macular Edema
Read More Genentech
Sensorion Completes Patient Recruitment in the P-IIa Study of SENS-401 for Residual Hearing Preservation After Cochlear Implantation
Read More Sensorion
Sirius Therapeutics Initiates P-I Trial of SRSD107 for Treating Thromboembolic Disorders
Read More Sirius Therapeutics
Qilu Pharmaceutical Publishes Results from the P-II Trial of QL1706 for Non-Small Cell Lung Cancer (NSCLC) in Signal Transduction and Targeted Therapy
Read More Qilu Pharmaceutical

Glenmark Signs a Collaboration Agreement with Jiangsu and 3D Medicines to Develop Envafolimab (KN035) for the Treatment of Cancer Indications
Read More: Glenmark
Synthekine Signs a Collaboration Agreement with Sanofi to Develop Selective IL-10 Agonists for the Treatment of Inflammatory Diseases
Read More: Synthekine & Sanofi
Agilent and Incyte Sign a Collaboration Agreement to Advance the Development of Companion Diagnostics
Read More Agilent & Incyte
Abbott Introduces Protality to Help Adults Lose Weight
Read More Abbott
Kiora Pharmaceuticals and Théa Open Innovation Have Collaborated to Develop and Commercialize KIO-301 for Retinal Diseases
Read More Kiora Pharmaceuticals & Théa Open Innovation
Inmagene Biopharmaceuticals to License HUTCHMED’s IMG-007 and IMG-004 Under the Agreement
Read More HUTCHMED & Inmagene Biopharmaceuticals

Ceva Santé to Acquire Scout Bio
Read More: Ceva Santé & Scout Bio
Merck Animal Health Introduces Allflex CleanVax Nozzles and Shields for Intranasal Vaccine Administration in Cattle
Read More Merck Animal Health

Orion Enters into a Collaborative Agreement with Newel Health to Develop ODD-403 for Chronic Pain
Read More: Orion & Newel Health

The US FDA Grants 510(k) Clearance to Copan Diagnostics’ Colibrí to Expand Automated ID/AST Capabilities
Read More: Copan Diagnostics
NuvoAir’s Air Next Spirometer Receives the US FDA’s 510(k) Clearance for In-Home Use
Read More NuvoAir
Boston Scientific’s Farapulse Pulsed Field Ablation System Receives the US FDA’s Approval for Atrial Fibrillation
Read More Boston Scientific
The US FDA Clears Hologic’s Genius Digital Diagnostics System to Identify Pre-Cancerous Lesions
Read More Hologic
Tags

Shivani was a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She was covering news related to Product approvals, clinical trial results, and updates. We can be contacted at connect@pharmashots.com.














