USGI Medical Receives FDA Approval to Conduct Pivotal Study of the POSE2.0 Incisionless Weight Loss Procedure for Primary Obesity
SAN CLEMENTE, Calif., Jan. 31, 2024 (GLOBE NEWSWIRE) -- USGI Medical, Inc. (USGI), the pioneer of incisionless weight loss procedures and devices, announced today that the United States Food and Drug Administration (FDA) has approved an Investigational Device Exemption (IDE) application to conduct a pivotal study of its POSE2.0® incisionless procedure for primary obesity. The clinical trial, known as INSPIRO™ (Incisionless Suture Plications (POSE2.0®) In a Randomized Obesity Study), will evaluate up to 186 participants at as many as seven U.S. sites and potentially other sites outside the U.S.
Source: USGI Medical
“The FDA’s action is another important milestone for USGI, and we are excited to move forward with a pivotal study to further validate POSE2.0®’s safety, durability and effectiveness in treating primary obesity,” said Mr. Arnold Podgorsky, Interim CEO of USGI Medical. “We appreciate the FDA’s support as we look to advance this potentially life-changing treatment option in the United States, which has already helped thousands of patients in other countries.”
There is a growing body of clinical data underscoring the positive impact that weight loss has on a range of comorbidities.1 Endoscopic gastric remodeling is an emerging approach that does not require the removal of the stomach, thus increasing access for more patients desiring less invasive options.
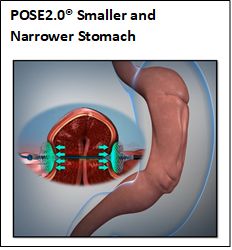
POSE2.0® uses USGI Medical’s Incisionless Operating Platform (IOP) to strategically place durable Snowshoe Suture Anchors in gastric tissue to both shorten and narrow the stomach. The procedure provides volumetric gastric restriction, like that achieved by a surgical sleeve gastrectomy, without surgically removing part of the stomach. Patients treated with the POSE2.0® procedure typically return to normal activities and lifestyles faster, compared with traditional laparoscopic and open abdominal surgery.
Barham Abu Dayyeh, M.D., MPH, of Mayo Clinic (Rochester, Minn.), is expected to lead the new INSPIRO pivotal study as Principal Investigator. Erik Wilson, M.D., Medical Director of Bariatric Surgery at the University of Texas McGovern Medical at Houston, will serve as Co-Principal Investigator.
Dr. Abu Dayyeh also served as Principal Investigator of the previously completed pilot study of POSE2.0® of 40 patients at four clinical centers that evaluated weight loss in adults suffering from obesity (body mass index [BMI] 35-40 kg/m²) who have an obesity-related comorbidity, such as diabetes or hypertension.
“This procedure holds promise, as it could prove to be a cost-effective, less burdensome therapeutic option with value added over existing pharmaceutical and surgical approaches,” added Podgorsky. “Marketing in the U.S. would be a game-changer for USGI, its investors and the doctor-patient community.”
About Obesity
Obesity is defined as abnormal or excessive fat accumulation that presents a risk to health. A body mass index (BMI) over 30 is considered obese. A relatively small and simple reduction in weight, for example, of around 5%, can improve patient outcomes and may act as a catalyst for further change, with sustainable weight loss achieved through a series of incremental weight loss steps. According to the Center for Disease Control and Prevention (CDC), the prevalence of obesity among U.S. adults today is approximately 40%, a rate that has grown significantly over the preceding decades, putting financial strains on the U.S. healthcare system with annual medical costs totaling more than $150 billion in U.S. dollars.
About USGI Medical, Inc.
USGI Medical develops technologies to enable Incisionless Surgery – the treatment of diseases through the natural passageways of the body. USGI's Incisionless Operating Platform (IOP) provides physicians the operating platform and specialized tools they need to perform procedures through a patient's mouth or other natural orifices. USGI has demonstrated the capability to suture GI tract tissue reliably and durably without an incision, opening the way to a new generation of treatments for a wide variety of acute and chronic diseases. Operating through the body's natural orifices offers promise for less pain, shorter hospital stays, reduced risk of wound infection along with no external scarring from abdominal incisions – and is rapidly becoming an option demanded by patients and healthcare providers. USGI offers surgeons and gastroenterologists the tools they need to offer millions of potential patients a less invasive option to surgery. For more information, go to http://www.usgimedical.com/.
The Incisionless Operating Platform, including the g-Cath EZ Delivery Catheter with Snowshoe® Suture Anchors, has both CE Mark and US 510(k) Clearance for tissue approximation. The safety and effectiveness of the device has not been established in the United States so as to permit marketing for the treatment of obesity. The device is considered an investigational device in the United States and is thereby limited by Federal law to investigational use for obesity treatment.
_____________________
1 Clin Gastroenterology Hepatology 2023 Jan;21(1):81-89. e4, Endoscopy 2021 Nov; 53(11):1169-1173, Endoscopy 2023 Nov; 55(11):1028-1034, Am J Gastroenterology. 2023 Jun 1; 118(6):983-990; Gastrointestinal Endoscopy. 2022 Sep;96(3):479-486.
For Further Information:
Media Contact:
Glenn Silver
Lazar-FINN Partners
glenn.silver@finnpartners.com
+1 646 871 8485
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/2f9f254f-581b-4f66-ae8e-b60d6c39c228
Source:- Globenewswire





