RenalGuard Therapy Receives FDA Breakthrough Device Designation for Prevention of Cardiac Surgery Associated AKI
MILFORD, Mass., Jan. 20, 2023 /PRNewswire/ -- CardioRenal Systems, Inc. announced today that the FDA granted Breakthrough Device Designation for its RenalGuard Therapy® device for the prevention of Acute Kidney Injury (AKI) in patients at risk for Cardiac Surgery Associated AKI (CSA-AKI).
"The high prevalence of acute kidney injury in cardiac surgery today is a well-known risk. We look forward to building further clinical validation that RenalGuard Therapy® can provide the solution to reduce the prevalence of CSA-AKI, the length and cost of hospitalization, and most importantly to improve patients' quality of life." Ilya Budik, CEO of CardioRenal Systems
The FDA's Breakthrough Device Designation is a federally legislated program is designed to expedite the review process and to facilitate the clinical trial development of devices that treat life-threatening conditions or irreversibly debilitating human disease or conditions. The designation is awarded when a device's preliminary clinical data suggest it might be more effective than the current standard of care on clinically significant endpoints of efficacy, safety, or patient quality of life where no approved or cleared alternatives exist.
Acute Kidney Injury affects millions of patients worldwide undergoing common hospitalizations. In the estimated 780,000 cardiac surgeries performed each year in the US, Europe and the Middle East, AKI is reported in up to 30% of cases, and is the most common major surgical complication. As many as 80% of patients have pre-existing risk factors that place them at high risk. AKI complicates patient recovery, adds significant time to ICU hospitalization, and is one of the strongest predictors of in-hospital and long-term mortality after surgery.
Ilya Budik, CardioRenal Systems' CEO commented, "We are thrilled to receive the Breakthrough Device Designation and appreciate all the hard work that our team put in to get us here. We are looking forward to working closely with the FDA and our partners to facilitate the initiation of the upcoming US pivotal study." Mr. Budik also added "The high prevalence of AKI in cardiac surgery today is a well-known risk. We look forward to building further clinical validation that RenalGuard Therapy® can provide the solution to reduce the prevalence of CSA-AKI, the length and cost of hospitalization, and most importantly to improve patients' quality of life."
About RenalGuard Therapy®
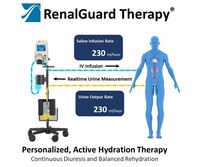
RenalGuard® is designed to protect the kidneys with personalized, active hydration. This is achieved by maximizing urine output, while balancing hydration via real-time urine output monitoring and IV infusion in a smart re-hydration system.
About CardioRenal Systems
CardioRenal Systems, Inc. is a Milford, Massachusetts based medical device company developing and marketing innovative technologies for AKI prevention and treatment in common hospitalizations including cardiac surgery, interventional cardiology, and ICU. The results from the KIDNEY Study, a 220-patient randomized, controlled clinical trial, demonstrated a 52% reduction in AKI with RenalGuard Therapy® vs. standard of care.
Contact Information:
Avi Wise
Business Development Manager
(508) 263-0243
352414@email4pr.com
www.renalguard.com
SOURCE CardioRenal Systems Inc.
Source:- PRNewswire