Rani Therapeutics Announces Positive Topline Results from Phase 1 Study of an Oral Anti-Interleukin 12/23 Antibody (RT-111)
SAN JOSE, Calif., Feb. 05, 2024 (GLOBE NEWSWIRE) -- Rani Therapeutics Holdings, Inc. (“Rani Therapeutics” or “Rani”) (Nasdaq: RANI), a clinical-stage biotherapeutics company focused on the oral delivery of biologics and drugs, today announced positive topline results from its Phase 1 clinical study of RT-111, a RaniPill® capsule containing an ustekinumab biosimilar, CT-P43. In the study, RT-111 was well-tolerated and delivered ustekinumab with high bioavailability.
“We are highly encouraged by the positive results from our Phase 1 study for RT-111 – our third successfully completed Phase 1 trial using RaniPill® technology. To our knowledge, RT-111 is the first ever oral monoclonal antibody to achieve high bioavailability in humans,” said Talat Imran, Chief Executive Officer of Rani. “These data provide clinical validation of our ability to successfully transform an injectable large molecule into an oral pill. Specifically for this program, we believe RT-111 has the potential to offer a highly differentiated dosing regimen for patients with psoriasis compared to both injectable biologics and oral small molecules and peptides. The success of the Phase 1 study of RT-111 marks another significant milestone for the Rani team, as we diligently work towards making oral biologics a reality for the millions of patients living with autoimmune conditions.”
Ustekinumab is a human IgG1қ monoclonal antibody that binds with specificity to the p40 protein subunit used by both the interleukin-12 and interleukin-23 (IL-12 and IL-23) cytokines. Currently, ustekinumab is available only as a subcutaneous injection (SC) and is marketed in the United States by Janssen as STELARA® for the treatment of moderate to severe plaque psoriasis, active psoriatic arthritis, moderate to severe Crohn’s disease, and moderate to severe ulcerative colitis, all of which have large unmet medical needs for oral treatment. Sales for STELARA were approximately $6.4 billion in the United States and approximately $9.7 billion worldwide in 2022.
Rani’s single-center, open label, Phase 1 study of RT-111 was conducted in Australia. The study evaluated the safety, tolerability, and pharmacokinetics (PK) of RT-111 in healthy adult volunteers. The study enrolled 20 participants each in RT-111 0.5mg and 0.75mg dose groups, and 15 participants in a STELARA (ustekinumab) 0.5mg subcutaneous (SC) injection group.
Phase 1 Topline Results
Pharmacokinetics
- Oral RT-111 delivered ustekinumab biosimilar in a dose proportional manner and with high bioavailability (estimated at 84% relative to subcutaneous injection).
- Oral RT-111 demonstrated a higher Cmax and shorter Tmax compared to ustekinumab delivered by SC injection (0.5mg).
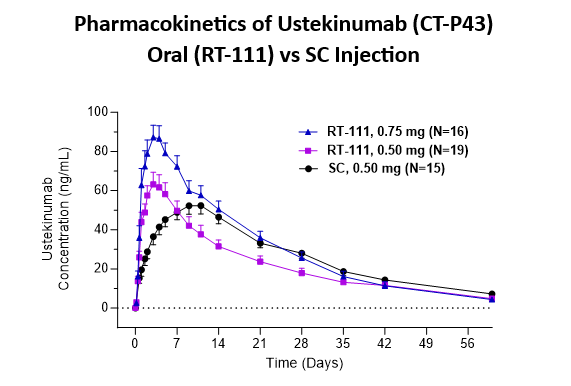
| Stelara® SC 0.50mg | RT-111 0.50mg | RT-111 0.75mg | |
| Cmax (ng/mL) | 56 ± 4 | 67 ± 7 | 92 ± 8 |
| Tmax (days) | 10 ± 0.8 | 3.1 ± 0.2* | 3.3 ± 0.2* |
| AUC (day*ng/mL) | 1,566 ± 130 | 1,315 ± 150 | 1,814 ± 165 |
Data are Mean ± SE from all subjects, including those with anti-drug antibodies. *p<0.0001 significantly different from SC group.
Safety and Tolerability
- RT-111 was well-tolerated by all participants in the two RT-111 groups, and no serious adverse events were observed in the study.
- There was no meaningful difference in incidence of anti-drug antibodies (ADA) via RaniPill® route of delivery, compared to STELARA® SC injection.
- No participants reported difficulty swallowing the capsule and capsule remnants passed from all participants without sequelae.
The ustekinumab biosimilar used in RT-111 is manufactured and supplied by Celltrion, Inc. (“Celltrion”) under Rani’s License and Supply Agreement with Celltrion announced in January 2023. Under that agreement, Celltrion exclusively supplies to Rani the ustekinumab biosimilar drug substance (CT-P43) required for RT-111. Rani is granted an exclusive license to use CT-P43 in the development and commercialization of RT-111, and Celltrion is granted a right of first negotiation to acquire worldwide rights to RT-111 following a Phase 1 clinical trial that meets its primary endpoint(s).
Conference Call
Rani will host a corresponding conference call and live webcast at 8:30 a.m. ET / 5:30 a.m. PT on February 5, 2024, to discuss the results from its Phase 1 clinical trial of RT-111. Individuals interested in listening to the live conference call may do so by using the webcast link in the “Investors” section of the company’s website at www.ranitherapeutics.com. A webcast replay will be available in the investor relations section on the company’s website for 90 days following the completion of the call.
About Rani Therapeutics
Rani Therapeutics is a clinical-stage biotherapeutics company focused on advancing technologies to enable the development of orally administered biologics and drugs. Rani has developed the RaniPill® capsule, which is a novel, proprietary and patented platform technology, intended to replace subcutaneous injection or intravenous infusion of biologics and drugs with oral dosing. Rani has successfully conducted several preclinical and clinical studies to evaluate safety, tolerability and bioavailability using RaniPill® capsule technology. For more information, visit ranitherapeutics.com.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things, the potential for RT-111 to be an alternative oral treatment option compared to burdensome injectables for autoimmune conditions, and the potential for Rani to make oral biologics a reality for millions of patients living with autoimmune conditions. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “intend” and “potential” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Rani’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties associated with Rani’s business in general and the other risks described in Rani’s filings with the Securities and Exchange Commission, including Rani’s annual report on Form 10-K for the year ended December 31, 2022, and subsequent filings and reports by Rani. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management’s assumptions and estimates as of such date. Rani undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Investor Contact:
investors@ranitherapeutics.com
Media Contact:
media@ranitherapeutics.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/5b0b63b1-6b9a-4593-95cd-cbb9a0592101
Source:- Rani Therapeutics





