Mylan Initiates Voluntary Nationwide Recall of Three Lots of Nizatidine Capsules, USP, Due to the Detection of Trace Amounts of NDMA (N-Nitrosodimethylamine) Impurity Found in the Active Phar
[caption id="attachment_9277" align="aligncenter" width="1079"] Press Release[/caption]
HERTFORDSHIRE, England, and?PITTSBURGH,?Jan. 8, 2020?/PRNewswire/ --?Mylan N.V.?(NASDAQ: MYL) today announced that its U.S. based Mylan Pharmaceuticals business is conducting a voluntary nationwide recall, to the consumer level, of three lots of Nizatidine Capsules, USP (including the 150mg and 300mg strengths). While Mylan has not received any reports of adverse events related to these batches to date, this product is being voluntarily recalled due to detected trace amounts of an impurity N-nitrosodimethylamine (NDMA) contained in the API Nizatidine, USP, manufactured by Solara Active Pharma Sciences Limited.
NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products and vegetables. NDMA has been classified as a probable human carcinogen (a substance that could cause cancer) according to the International Agency for Research on Cancer (IARC).
The finished products are manufactured by Mylan Pharmaceuticals Inc. These batches were distributed nationwide to wholesalers, mail order pharmacies, retail pharmacies, and a distributor between?June 2017?and?August 2018. The recalled batches are as follows:
Press Release[/caption]
HERTFORDSHIRE, England, and?PITTSBURGH,?Jan. 8, 2020?/PRNewswire/ --?Mylan N.V.?(NASDAQ: MYL) today announced that its U.S. based Mylan Pharmaceuticals business is conducting a voluntary nationwide recall, to the consumer level, of three lots of Nizatidine Capsules, USP (including the 150mg and 300mg strengths). While Mylan has not received any reports of adverse events related to these batches to date, this product is being voluntarily recalled due to detected trace amounts of an impurity N-nitrosodimethylamine (NDMA) contained in the API Nizatidine, USP, manufactured by Solara Active Pharma Sciences Limited.
NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products and vegetables. NDMA has been classified as a probable human carcinogen (a substance that could cause cancer) according to the International Agency for Research on Cancer (IARC).
The finished products are manufactured by Mylan Pharmaceuticals Inc. These batches were distributed nationwide to wholesalers, mail order pharmacies, retail pharmacies, and a distributor between?June 2017?and?August 2018. The recalled batches are as follows:
Nizatidine is indicated for the short-term treatment (up to 8 weeks) of active duodenal ulcers and active benign gastric ulcers, as maintenance therapy for duodenal ulcer patients for up to one year, and for up to 12 weeks for the treatment of endoscopically diagnosed esophagitis and associated heartburn due to gastroesophageal reflux disease (GERD).
Mylan is notifying its distributors and customers by letter and is arranging for return of all recalled products. Wholesalers, retailers and consumers that are in possession of recalled product should contact Stericycle at 888-628-0727 for the return of the recalled product. Normal business hours are Monday through Friday?8 a.m. to 5 p.m. EST.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these drug products.
 SOURCE Mylan N.V.
SOURCE Mylan N.V.

 Press Release[/caption]
HERTFORDSHIRE, England, and?PITTSBURGH,?Jan. 8, 2020?/PRNewswire/ --?Mylan N.V.?(NASDAQ: MYL) today announced that its U.S. based Mylan Pharmaceuticals business is conducting a voluntary nationwide recall, to the consumer level, of three lots of Nizatidine Capsules, USP (including the 150mg and 300mg strengths). While Mylan has not received any reports of adverse events related to these batches to date, this product is being voluntarily recalled due to detected trace amounts of an impurity N-nitrosodimethylamine (NDMA) contained in the API Nizatidine, USP, manufactured by Solara Active Pharma Sciences Limited.
NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products and vegetables. NDMA has been classified as a probable human carcinogen (a substance that could cause cancer) according to the International Agency for Research on Cancer (IARC).
The finished products are manufactured by Mylan Pharmaceuticals Inc. These batches were distributed nationwide to wholesalers, mail order pharmacies, retail pharmacies, and a distributor between?June 2017?and?August 2018. The recalled batches are as follows:
Press Release[/caption]
HERTFORDSHIRE, England, and?PITTSBURGH,?Jan. 8, 2020?/PRNewswire/ --?Mylan N.V.?(NASDAQ: MYL) today announced that its U.S. based Mylan Pharmaceuticals business is conducting a voluntary nationwide recall, to the consumer level, of three lots of Nizatidine Capsules, USP (including the 150mg and 300mg strengths). While Mylan has not received any reports of adverse events related to these batches to date, this product is being voluntarily recalled due to detected trace amounts of an impurity N-nitrosodimethylamine (NDMA) contained in the API Nizatidine, USP, manufactured by Solara Active Pharma Sciences Limited.
NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products and vegetables. NDMA has been classified as a probable human carcinogen (a substance that could cause cancer) according to the International Agency for Research on Cancer (IARC).
The finished products are manufactured by Mylan Pharmaceuticals Inc. These batches were distributed nationwide to wholesalers, mail order pharmacies, retail pharmacies, and a distributor between?June 2017?and?August 2018. The recalled batches are as follows:
|
NDC |
Product Description |
Strength |
Size |
Lot Number |
Expiry |
|
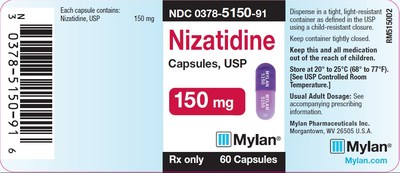
0378-5150-91 |
Nizatidine Capsules, USP |
150mg |
Bottles of 60 |
3086746 |
May 2020 |
|
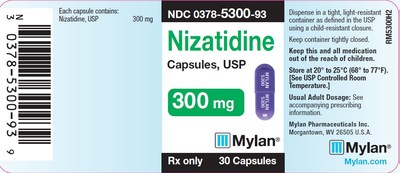
0378-5300-93 |
Nizatidine Capsules, USP |
300mg |
Bottles of 30 |
3082876 |
Jan 2020 |
|
0378-5300-93 |
Nizatidine Capsules, USP |
300mg |
Bottles of 30 |
3082877 |
Jan 2020 |
- Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax. Complete and submit the report Online:?www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form?www.fda.gov/MedWatch/getforms.htm?or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.



For further information: Christine Waller (Media), 724.514.1968; Melissa Trombetta (Investors), 724.514.1813





