Lilly's bamlanivimab and etesevimab together reduced hospitalizations and death in Phase 3 trial for early COVID-19
 INDIANAPOLIS,?March 10, 2021?/PRNewswire/ --?Eli Lilly and Company?(NYSE: LLY) today announced new data from the randomized, double-blind, placebo-controlled BLAZE-1 Phase 3 study, demonstrating bamlanivimab (LY-CoV555) 700 mg and etesevimab (LY-CoV016) 1400 mg together significantly reduced COVID-19 related hospitalizations and deaths ("events") in high-risk patients recently diagnosed with COVID-19. These results provide additional efficacy and safety data that support the use of the dose recently granted both Emergency Use Authorization by the?U.S. Food and Drug Administration?(FDA) and a positive scientific opinion by the?European Medicines Agency's?(EMA)?Committee for Medicinal Products?for Human Use (CHMP).
This new Phase 3 cohort of BLAZE-1 included 769 high-risk patients, aged 12 and older with mild to moderate COVID-19 (therapy: n=511; placebo: n=258). There were four events in patients taking bamlanivimab with etesevimab and 15 events in patients taking placebo, representing an 87 percent risk reduction (p<0.0001). Bamlanivimab and etesevimab together also demonstrated statistically significant improvements on key secondary endpoints. These results are consistent with those seen in other data sets from BLAZE-1: in the previous Phase 3 cohort, bamlanivimab 2800 mg with etesevimab 2800 mg reduced the risk of hospitalizations and deaths by 70 percent and in the Phase 2 cohort, bamlanivimab alone reduced the risk of hospitalizations and ER visits by approximately 70 percent. The viral load reductions were also consistent with what was observed in the previous Phase 3 cohort of the study.
In this new Phase 3 cohort, there were four deaths total, all of which were deemed related to COVID-19 and all of which occurred in patients taking placebo; no deaths occurred in patients receiving treatment with bamlanivimab and etesevimab together. Across the two Phase 3 cohorts of the study that have been analyzed to date, there have been no deaths in patients receiving treatment with bamlanivimab and etesevimab together, and 14 deaths in patients receiving placebo, 13 of which were deemed COVID-19 related. In this data set, the safety profile of bamlanivimab and etesevimab together was consistent with observations from other Phase 1, Phase 2 and Phase 3 trials evaluating these antibodies.
INDIANAPOLIS,?March 10, 2021?/PRNewswire/ --?Eli Lilly and Company?(NYSE: LLY) today announced new data from the randomized, double-blind, placebo-controlled BLAZE-1 Phase 3 study, demonstrating bamlanivimab (LY-CoV555) 700 mg and etesevimab (LY-CoV016) 1400 mg together significantly reduced COVID-19 related hospitalizations and deaths ("events") in high-risk patients recently diagnosed with COVID-19. These results provide additional efficacy and safety data that support the use of the dose recently granted both Emergency Use Authorization by the?U.S. Food and Drug Administration?(FDA) and a positive scientific opinion by the?European Medicines Agency's?(EMA)?Committee for Medicinal Products?for Human Use (CHMP).
This new Phase 3 cohort of BLAZE-1 included 769 high-risk patients, aged 12 and older with mild to moderate COVID-19 (therapy: n=511; placebo: n=258). There were four events in patients taking bamlanivimab with etesevimab and 15 events in patients taking placebo, representing an 87 percent risk reduction (p<0.0001). Bamlanivimab and etesevimab together also demonstrated statistically significant improvements on key secondary endpoints. These results are consistent with those seen in other data sets from BLAZE-1: in the previous Phase 3 cohort, bamlanivimab 2800 mg with etesevimab 2800 mg reduced the risk of hospitalizations and deaths by 70 percent and in the Phase 2 cohort, bamlanivimab alone reduced the risk of hospitalizations and ER visits by approximately 70 percent. The viral load reductions were also consistent with what was observed in the previous Phase 3 cohort of the study.
In this new Phase 3 cohort, there were four deaths total, all of which were deemed related to COVID-19 and all of which occurred in patients taking placebo; no deaths occurred in patients receiving treatment with bamlanivimab and etesevimab together. Across the two Phase 3 cohorts of the study that have been analyzed to date, there have been no deaths in patients receiving treatment with bamlanivimab and etesevimab together, and 14 deaths in patients receiving placebo, 13 of which were deemed COVID-19 related. In this data set, the safety profile of bamlanivimab and etesevimab together was consistent with observations from other Phase 1, Phase 2 and Phase 3 trials evaluating these antibodies.
? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ?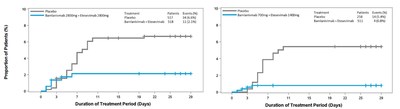 Figure 1: Time to COVID-19 related hospitalization
Figure 1: Time to COVID-19 related hospitalization
"These positive results reinforce our previous findings and support the authorized dose of bamlanivimab 700 mg with etesevimab 1400 mg.?These compelling data ? in addition to the recent EUA from FDA, the CHMP decision from EMA and the recommendation for the therapy in the?National Institutes of Health's?COVID-19 Treatment Guidelines ? give healthcare providers additional information regarding the use of bamlanivimab and etesevimab together as a potentially life-saving treatment to help those most at risk for severe complications of COVID-19," said?Daniel Skovronsky, M.D., Ph.D.,?Lilly's chief scientific officer and president of?Lilly Research Laboratories.?"The consistent results observed in multiple cohorts of this trial over several months, even as new strains of COVID-19 have emerged, indicate bamlanivimab with etesevimab maintains its effects against a range of variants, particularly those circulating in the?U.S."
Lilly?continues to engage with global regulators to make bamlanivimab alone and bamlanivimab and etesevimab together available around the world. Bamlanivimab alone and bamlanivimab with etesevimab together are authorized under special/emergency pathways, in the context of the pandemic, in the?U.S.?and the?European Union. In addition, bamlanivimab alone is authorized for emergency use in?Canada,?Panama,?Kuwait, the?UAE,?Israel,?Rwanda,?Morocco?and?numerous other countries. Through?Lilly's work with the?Bill & Melinda Gates Foundation,?Lilly?is providing doses of bamlanivimab free of charge in?Rwanda?and?Morocco.
For more information about the use of bamlanivimab alone or bamlanivimab and etesevimab together for the treatment of mild to moderate COVID-19 in high-risk patients under the?FDA's?emergency use authorization, contact?Lilly's 24-hour support line at 1-855-LillyC19 (1-855-545-5921). Patients and physicians can visit?lillyantibody.com?to learn more, including how to find a potential treatment location.
For media resources, including product images and fact sheets, please click?here.
Important Information about bamlanivimab alone and bamlanivimab and etesevimab together
Bamlanivimab and etesevimab together and bamlanivimab alone have not been approved by the FDA for any use. It is not known if bamlanivimab and etesevimab together or bamlanivimab alone are?safe and effective for the treatment of COVID-19.
Bamlanivimab and etesevimab together and bamlanivimab alone are?authorized under Emergency Use Authorization only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use under Section 564(b)(1) of the Act, 21 U.S.C ? 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Healthcare providers should review the Fact Sheet for information on the authorized use of bamlanivimab and etesevimab together and bamlanivimab alone and mandatory requirements of the EUA. Please see the?FDA Letter of Authorization,?Fact Sheet for Healthcare Providers, and Fact Sheet for Patients, Parents and Caregivers (English) (Spanish) for bamlanivimab and etesevimab together. Please see the?FDA Letter of Authorization,?Fact Sheet for Healthcare Providers, and Fact Sheet for Patients, Parents and Caregivers?(English) (Spanish) for bamlanivimab alone.
Authorized Use and Important Safety Information
Bamlanivimab and etesevimab together and bamlanivimab alone are?authorized for use under EUA for treatment of mild to moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg)?with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progressing to severe COVID-19 and/or hospitalization.
Limitations of Authorized Use
 Figure 1: Time to COVID-19 related hospitalization
Figure 1: Time to COVID-19 related hospitalization
- Bamlanivimab and etesevimab together and bamlanivimab alone are not authorized for use in patients:
- who are hospitalized due to COVID-19, OR
- who require oxygen therapy due to COVID-19, OR
- who require an increase in baseline oxygen flow rate due to COVID-19 in those on chronic oxygen therapy due to underlying non-COVID-19 related comorbidity.
- Treatment with bamlanivimab?and etesevimab together has not been studied in patients hospitalized due to COVID-19. Benefit of treatment with bamlanivimab alone has not been observed in patients hospitalized due to COVID-19. Monoclonal antibodies, such as bamlanivimab?and etesevimab, may be associated with worse clinical outcomes when administered to hospitalized patients with COVID-19 requiring high flow oxygen or mechanical ventilation.
- fever, difficulty breathing, reduced oxygen saturation, chills, fatigue, arrhythmia (e.g. atrial fibrillation, sinus tachycardia, bradycardia), chest pain or discomfort, weakness, altered mental status, nausea, headache, bronchospasm, hypotension, hypertension, angioedema, throat irritation, rash including urticaria, pruritus, myalgia, dizziness, and diaphoresis.
| Refer to: | Molly McCully;?mccully_molly@lilly.com; 317-478-5423 (Media) |
| Dani Barnhizer;?dbarnhizer@lilly.com; 317-607-6119 (Media) | |
| Kevin Hern;?hern_kevin_r@lilly.com; 317-277-1838 (Investors) |





