Akebia Presents Results from its INNO2VATE Global Phase 3 Program; Demonstrated Efficacy and Cardiovascular Safety of Vadadustat for the Treatment of Anemia due to Chronic Kidney Disease in A
 CAMBRIDGE, Mass.,?Oct. 22, 2020?/PRNewswire/ --?Akebia Therapeutics, Inc.?(Nasdaq: AKBA) today announced the presentation of clinical data from its global INNO2VATE Phase 3 program, which demonstrated the efficacy and cardiovascular safety of vadadustat for the treatment of anemia due to chronic kidney disease (CKD) in adult patients on dialysis, at?American Society of Nephrology Kidney Week?2020?Reimagined (ASN Kidney Week). Vadadustat is Akebia's investigational oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI) in development for the treatment of anemia due to CKD.
"The data presented today build on the positive top-line efficacy and safety results from INNO2VATE that were previously reported in May. More specifically, vadadustat's cardiovascular safety profile in dialysis patients is further reinforced by newly presented data clearly showing vadadustat achieved non-inferiority to darbepoetin alfa on MACE, expanded MACE, cardiovascular MACE, cardiovascular mortality, and all-cause mortality. These results were also consistent across multiple pre-specified populations,"?said?Kai-Uwe Eckardt, M.D., Professor of Medicine and Head of the?Department of Nephrology and Medical Intensive Care Medicine?at the Charit? in?Berlin?and Co-Chair of the independent?Executive Steering Committee?for INNO2VATE. "Left untreated, anemia?in dialysis patients results in high transfusion requirements and severely reduces a patient's quality of life. The INNO2VATE results demonstrate that vadadustat could represent an attractive new oral treatment option for patients new to and already established on dialysis, upon approval."
Results from INNO2VATE are being presented today at?ASN Kidney Week during a presentation titled, "Global Phase 3 Clinical Trials of Vadadustat vs Darbepoetin Alfa for Treatment of Anemia in Patients with Dialysis-Dependent Chronic Kidney Disease"?(Abstract TH-OR01).
Highlights of the INNO2VATE ASN Kidney Week Presentation:
Efficacy:
Safety:
CAMBRIDGE, Mass.,?Oct. 22, 2020?/PRNewswire/ --?Akebia Therapeutics, Inc.?(Nasdaq: AKBA) today announced the presentation of clinical data from its global INNO2VATE Phase 3 program, which demonstrated the efficacy and cardiovascular safety of vadadustat for the treatment of anemia due to chronic kidney disease (CKD) in adult patients on dialysis, at?American Society of Nephrology Kidney Week?2020?Reimagined (ASN Kidney Week). Vadadustat is Akebia's investigational oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI) in development for the treatment of anemia due to CKD.
"The data presented today build on the positive top-line efficacy and safety results from INNO2VATE that were previously reported in May. More specifically, vadadustat's cardiovascular safety profile in dialysis patients is further reinforced by newly presented data clearly showing vadadustat achieved non-inferiority to darbepoetin alfa on MACE, expanded MACE, cardiovascular MACE, cardiovascular mortality, and all-cause mortality. These results were also consistent across multiple pre-specified populations,"?said?Kai-Uwe Eckardt, M.D., Professor of Medicine and Head of the?Department of Nephrology and Medical Intensive Care Medicine?at the Charit? in?Berlin?and Co-Chair of the independent?Executive Steering Committee?for INNO2VATE. "Left untreated, anemia?in dialysis patients results in high transfusion requirements and severely reduces a patient's quality of life. The INNO2VATE results demonstrate that vadadustat could represent an attractive new oral treatment option for patients new to and already established on dialysis, upon approval."
Results from INNO2VATE are being presented today at?ASN Kidney Week during a presentation titled, "Global Phase 3 Clinical Trials of Vadadustat vs Darbepoetin Alfa for Treatment of Anemia in Patients with Dialysis-Dependent Chronic Kidney Disease"?(Abstract TH-OR01).
Highlights of the INNO2VATE ASN Kidney Week Presentation:
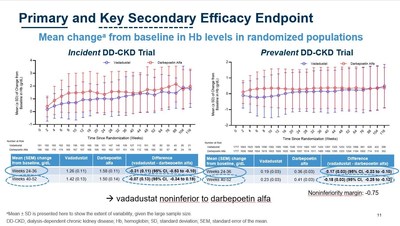
Efficacy:
Safety:
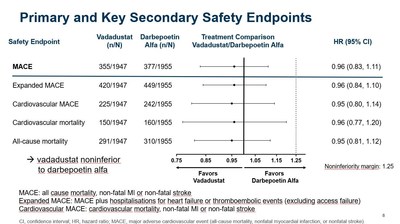
- As previously reported, vadadustat achieved the primary safety endpoint of the INNO2VATE program, defined as non-inferiority of vadadustat versus darbepoetin alfa in time to first occurrence of a major adverse cardiovascular event (MACE), which is the composite of all-cause mortality, non-fatal myocardial infarction (MI), or non-fatal stroke across both INNO2VATE studies.
- INNO2VATE results on key secondary safety endpoints were clear and consistent. Vadadustat demonstrated non-inferiority to darbepoetin alfa in analyses of expanded MACE, cardiovascular MACE, cardiovascular mortality, and all-cause mortality.
- The incidence of treatment emergent adverse events during the incident dialysis patient (Correction/Conversion)?study in vadadustat treated patients was 83.8% and 85.5% in darbepoetin alfa treated patients. During the study, the most common treatment emergent adverse events reported in vadadustat/darbepoetin alfa treated patients were hypertension (16.2%/ 12.9%) and diarrhea (10.1%/ 9.7%). Serious treatment emergent adverse events were lower in vadadustat treated patients at 49.7% compared to 56.5% for darbepoetin alfa treated patients. The incidence of treatment emergent adverse events during the prevalent dialysis patient (Conversion)?study in the vadadustat treated patients was 88.3%, and 89.3% in darbepoetin alfa treated patients. During the study, the most common treatment emergent adverse events reported in vadadustat/darbepoetin alfa treated patients were diarrhea (13.0%/ 10.1%), pneumonia (11.0%/ 9.7%), hypertension (10.6%/ 13.8%), and hyperkalemia (9.0%/ 10.8%). Serious treatment emergent adverse events were slightly lower for vadadustat treated patients at 55.0% and 58.3% for darbepoetin alfa-treated patients.