Abbott Vascular Recalls NC Trek RX and NC Traveler RX Coronary Dilatation Catheters Due to Failure of Balloon (diameter 4.0mm, 4.5mm and 5.00mm) to Deflate
[caption id="attachment_9277" align="aligncenter" width="747"] Press Release[/caption]
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Recalled Product:
Press Release[/caption]
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Recalled Product:
Additional Resources
NC TRAVLER Coronary Dilatation Catheter (4.5 X8MM) - Recall Database Entry
NC TRAVLER Coronary Dilatation Catheter (4.5 X 12MM) - Recall Database Entry
NC TRAVLER Coronary Dilatation Catheter (4.0 X 12MM) ? Recall Database Entry
NC TRAVLER Coronary Dilatation Catheter (4.0 X 8MM) ? Recall Database Entry
NC TREK Coronary Dilatation Catheter (5.00 X 20MM BDC) ? Recall Database Entry
NC TREK Coronary Dilatation Catheter (5.00 X 15MM BDC) ? Recall Database Entry
NC TREK Coronary Dilatation Catheter (5.00 X 12MM BDC) ? Recall Database Entry
NC TREK Coronary Dilatation Catheter (5.00 X 8MM BDC) ? Recall Database Entry
NC TREK Coronary Dilatation Catheter (4.50 X 20MM BDC) - Recall Database Entry
NC TREK Coronary Dilatation Catheter (4.50 X 15MM BDC) ? Recall Database Entry
Abbott Vascular Urgent Field NoticeExternal Link Disclaimer
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experience using these devices to?MedWatch: The FDA Safety Information and Adverse Event Reporting Program?either online, by regular mail or by FAX.
 Press Release[/caption]
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Recalled Product:
Press Release[/caption]
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Recalled Product:
- NC Trek RX Coronary Dilatation Catheter and NC Traveler RX Coronary Dilatation Catheters Models: Balloon diameters 4.0mm, 4.5mm and 5.0mm Lots:?See a full list of affected devices
- Distribution Dates: August 2019 to January 2020
- Devices Recalled in the U.S.: 13,891
- Date Initiated by Firm: January 29, 2020

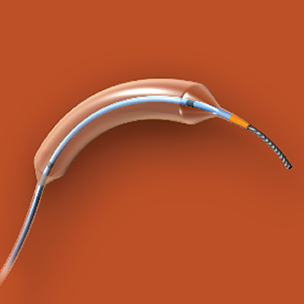
Image of the Abbott Vascular NC Trek RX Coronary Dilatation Catheter
Device Use: Coronary dilation catheters are used to open clogged blood vessels to improve blood flow to the heart. The NC TREK RX Coronary Dilatation Catheter is indicated for use in the following cardiac (heart) procedures:- balloon dilatation of the abnormal narrowing portion of a?coronary artery or bypass graft, for the purpose of improving?myocardial perfusionExternal Link Disclaimer.
- balloon dilatation of a coronary artery occlusion, to restore coronary flow in patients who have had a type of heart attack, known as?myocardial infarction?? specifically patients with ST-segment elevation, which refers to the flat section of an electrocardiogram (ECG) reading and represents the interval between heartbeats.
- balloon dilatation of a stent after implantation (balloon models 4.00 mm ? 5.00 mm only).
- Patients who undergo cardiac procedures involving the NC Trek RX Coronary Dilatation Catheter and NC Traveler RX Coronary Dilatation Catheters.
- Health care providers and hospitals using the NC Trek RX Coronary Dilatation Catheter and NC Traveler RX Coronary Dilatation Catheters.
- Immediately stop using affected devices from the lots affected by this recall.
- Review your inventory, complete, and return the attached Effectiveness Check Form.
- Return all unused affected product to Abbott Vascular.
- Share the notification with other relevant personnel in your organization.
| Device Identifier/GTIN | Device Description | Part Number | Lot Numbers |
| 08717648152054 | NC TREK RX 4.00 X 8MM BDC | 1012453-08 | 90731G1 90808G1 90826G1 90906G1 90921G1 90928G1 91003G1 91017G1 91106G1 |
| 08717648152061 | NC TREK RX 4.00 X 12MM BDC | 1012453-12 | 90730G1 90731G1 90818G1 90822G1 90905G1 90918G1 90919G1 90927G1 91001G1 91015G1 91025G1 91101G1 |
| 08717648152078 | NC TREK RX 4.00 X 15MM BDC | 1012453-15 | 90815G1 90816G1 90828G1 90904G1 90920G1 90926G1 91008G1 91009G1 91020G1 91026G1 91109G1 91117G1 |
| 08717648152085 | NC TREK RX 4.00 X 20MM BDC | 1012453-20 | 90727G1 90830G1 90923G1 91004G1 91028G1 |
| 08717648152092 | NC TREK RX 4.50 X 8MM BDC | 1012454-08 | 90801G1 90812G1 90818G1 90818G2 90904G1 90912G1 90925G1 91010G2 91022G1 91025G1 91101G1 |
| 08717648152108 | NC TREK RX 4.50 X 12MM BDC | 1012454-12 | 90731G1 90805G1 90817G1 90818G1 90904G1 90906G1 90912G1 90922G1 91015G1 91016G1 91105G1 |
| 08717648152115 | NC TREK RX 4.50 X 15MM BDC | 1012454-15 | 90819G1 90819G2 90916G1 90916G2 90927G1 90930G1 91031G1 |
| 08717648152122 | NC TREK RX 4.50 X 20MM BDC | 1012454-20 | 90801G1 90809G1 91018G1 91021G1 |
| 08717648152139 | NC TREK RX 5.00 X 8MM BDC | 1012455-08 | 90918G1 90930G1 91001G1 |
| 08717648152146 | NC TREK RX 5.00 X 12MM BDC | 1012455-12 | 90918G1 90926G1 90930G1 91031G1 |
| 08717648152153 | NC TREK RX 5.00 X 15MM BDC | 1012455-15 | 90806G1 90806G2 91022G1 91025G1 |
| 08717648152160 | NC TREK RX 5.00 X 20MM BDC | 1012455-20 | 91010G1 91026G1 |
| 08717648195983 | NC Traveler RX 4.0 X 8MM | 1013157-08 | 91010G1 |
| 08717648195990 | NC Traveler RX 4.0 X 12MM | 1013157-12 | 90812G1 |
| 08717648196003 | NC Traveler RX 4.0 X 15MM | 1013157-15 | 91102G1 |
| 08717648196027 | NC Traveler RX 4.5 X 8MM | 1013158-08 | 90812G1 |
| 08717648196034 | NC Traveler RX 4.5 X 12MM | 1013158-12 | 90813G1 |





