New Drug Designations: July 2025
Shots:
- PharmaShots’ Designation Report offers a concise overview of the latest drug and device designations granted by major regulatory authorities, including the FDA, EMA, MHLW, Health Canada, and NMPA.
- The July 2025 edition covers designations awarded to 47 drugs and 3 medical devices, comprising 23 small molecules, 9 biologics, 8 cell and gene therapies, and 3 medical devices, among others.
- Key highlights this month include BeOne Medicines’ BGB-16673 receiving both Prime and Orphan Drug status
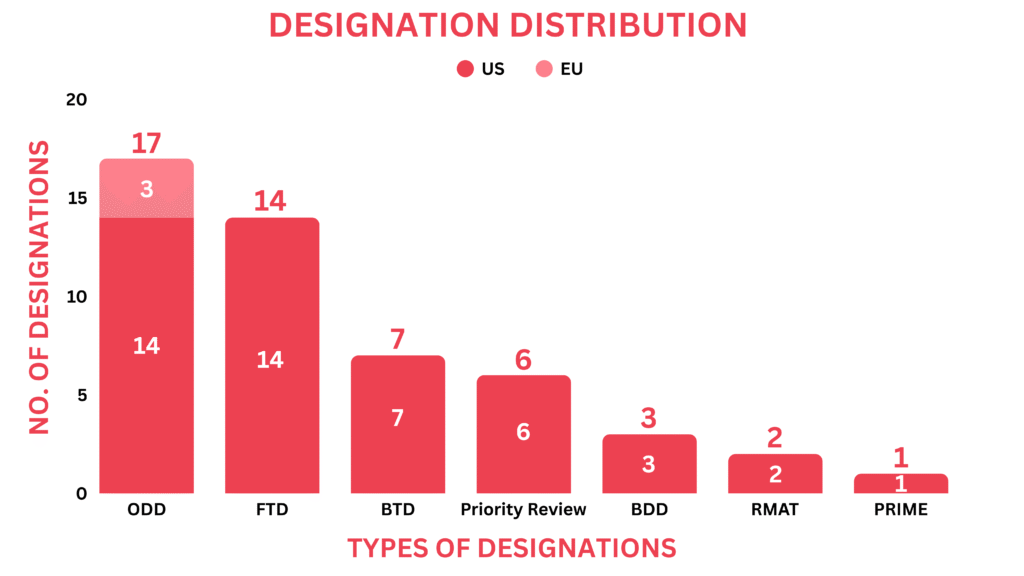

Drugs receiving orphan drug designation by global regulatory bodies echoed as small molecules, biologics, and gene therapies. Around 18 drugs received the orphan drug designation across multiple indications.
A Quick Look
Gene Therapies: KLTO 202 (Klotho Neurosciences) for Amyotrophic Lateral Sclerosis, PBGENE-DMD (Precision BioSciences) for Duchenne Muscular Dystrophy, Small Molecules: BGB-16673 (BeOne Medicines) for Waldenstrom’s macroglobulinemia, Quemliclustat (Arcus Biosciences) for Pancreatic Cancer, FF-10832 (FUJIFILM Pharmaceuticals) for Biliary Tract Cancer, ACD440 (AlzeCure Pharma) for Peripheral Neuropathic Pain, ENV-101 (Endeavor BioMedicines) for Idiopathic Pulmonary Fibrosis, VT3989 (Vivace Therapeutics) for Mesothelioma, GLIX1 (Hemispherian) for Malignant Glioma, SH-110 (Shorla Oncology) for Glioma, NX-5948 (Nurix Therapeutics) for Waldenstrom’s Macroglobulinemia, MP1032 (MetrioPharm) for Duchenne Muscular Dystrophy, Biologics: ICT01 (ImCheck Therapeutics) for Acute Myeloid Leukemia, MB-101(Mustang Bio) for Astrocytomas and Glioblastoma, ADRX-0405 (Adcentrx Therapeutics) for Gastric Cancer, SAR446523 (Sanofi) for R/R Multiple Myeloma, Peptide: AMFX-200 (Amphix Bio) for Acute Spinal Cord Injury, Oncolytic Virus: CAN-2409 (Candel Therapeutics) for Pancreatic Cancer

Around 14 drugs received the Fast Track Designation as gene therapy, small molecules, biologics, recombinant protein, and oncolytic virus.
A Quick Look
Gene Therapies: SAR446597 (Sanofi) for Geographic Atrophy/AMD, SGT-501 (Solid Biosciences) for Catecholaminergic Polymorphic Ventricular Tachycardia, Small Molecules: Nuvisertib (Sumitomo Pharma) for Myelofibrosis, VS-7375 (Verastem Oncology) for KRAS G12D-mutated Pancreatic Cancer, ZEN-3694 (Zenith Epigenetics) for NUT carcinoma, Ateganosine (MAIA Biotechnology) for NSCLC, SAP-001 (Shanton Pharma) for Hyperuricemia/gout, TRE-515 (Trethera) for Metastatic Prostate Cancer, DSB2455 (Duke Street Bio) for Brain Metastases/TNBC, ABS-1230 (Actio Biosciences) for KCNT1-related Epilepsy, Biologic: DB-1310 (Yingen Biotech) for Non-squamous NSCLC, PMN310 (ProMIS Neurosciences) for Alzheimer’s Disease, Recombinant Protein: Rezpegaldesleukin (Nektar Therapeutics) for Alopecia Areata, Oncolytic Virus: CLD-201 (Calidi Biotherapeutics) for Soft Tissue Sarcoma

Regulatory bodies designated seven drugs with the Breakthrough Therapy Designation, ranging from small molecules and gene therapies to biologics and Antibody Oligonucleotide Conjugate.
A Quick Look
Gene Therapy: LX2006 (Lexeo Therapeutics) for Friedreich’s ataxia, Small Molecules: Relutrigine (Praxis) for Epilepsy, Daraxonrasib (Revolution Medicines) for Pancreatic Ductal Adenocarcinoma, TSND-201 (Transcend Therapeutics) for PTSD, Biologics: Enhertu (Daiichi Sankyo & AstraZeneca) for Breast Cancer, Imfinzi (AstraZeneca) for Stages II, III, IVA G/GEJ Cancer, Antibody Oligonucleotide Conjugate: Delpacibart zotadirsen (Avidity Biosciences) for Duchenne Muscular Dystrophy

Six drugs, ranging from recombinant proteins and small molecules to gene therapy and biologics were given priority review.
A Quick Look
Gene Therapy: Tabelecleucel (Atara Biotherapeutics) for EBV+ Post-transplant lymphoproliferative disease, Recombinant Protein: Tividenofusp alfa (Denali Therapeutics) for Hunter syndrome, Winrevair (Merck) for Pulmonary arterial hypertension, Small Molecules: TAR-200 (Johnson & Johnson) for Non-muscle invasive bladder cancer, Addyi (Sprout Pharmaceuticals) for Low sexual desire, Biologics: Imfinzi (AstraZeneca) for Stages II, III, IVA G/GEJ Cancer

BeOne Medicines’ BGB-16673 received the Prime Designation
A Quick Look
Small Molecule: BGB-16673 (BeOne Medicines) for Waldenstrom’s macroglobulinemia

Two gene therapy drugs received the regenerative medicine advanced therapy designation.
A Quick Look
Gene Therapies: GNSC-001 (Genascence) for knee osteoarthritis, RP-A601 (Rocket Pharmaceuticals) for PKP2-arrhythmogenic cardiomyopathy

Three devices received the breakthrough device designation by the US FDA.
A Quick Look
A Quick Look
SonoClear System (SonoClear) for Intracranial ultrasound, ArteraAI Prostate (Artera) for Prostate cancer (decision-making), DecisionDx-Melanoma test (Castle Biosciences) for Melanoma risk stratification
Related Post: New Drug Designations: June 2025




