EMA Marketing Authorization of New Drugs in June 2025
Shots:
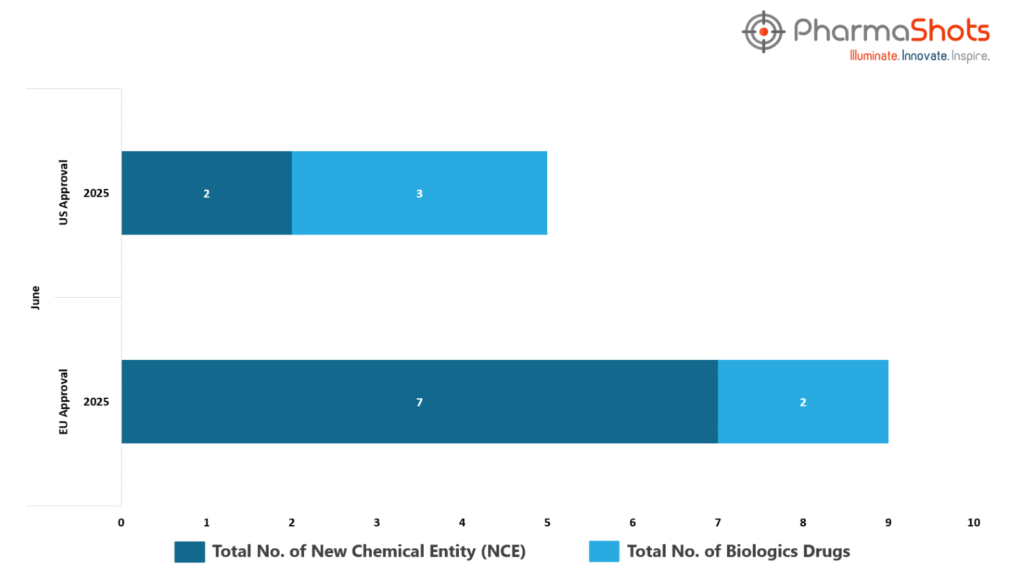
- The EMA’s CHMP has granted positive opinions and approvals to 2 Biologics and 6 new chemical entities in June 2025, leading to treatments for patients and advances in the healthcare industry
- The major highlighted drug was SpringWorks Therapeutics’ Ogsiveo to Treat Desmoid Tumours
- PharmaShots has compiled a list of 8 drugs that have been granted positive opinions and approvals by the EC, respectively
Company: Italfarmaco
Product: Duvyzat
Active Ingredient: Givinostat
Disease: Duchenne Muscular Dystrophy
Date: Jun 06, 2025
Shots:
- The EC has granted conditional approval to Duvyzat (givinostat) for the treatment of ambulant DMD pts (≥6yrs.) on corticosteroids, regardless of underlying genetic mutation in all 30 EEA states
- Approval was based on the P-III (EPIDYS) study assessing Duvyzat (BID) vs PBO in ambulant DMD boys (n=179) on corticosteroid therapy
- The trial met its 1EP by improving four-stair climb completion time, with benefits seen in 2EPs (NSAA & MRI fat infiltration) & a 40% decrease in cumulative NSAA item loss; data was published in The Lancet Neurology. Ongoing extension trial data showed delay in DMD progression, with median ambulation loss at 18.1 vs 15.2yrs. for controls
Company: Averoa
Product: Xoanacyl
Active Ingredient: Ferric Citrate Coordination Complex
Disease: Chronic Kidney Disease
Date: Jun 16, 2025
Shots:
- The EC has approved Xoanacyl (ferric citrate complex) for chronic kidney disease; UK’s MAA was filed via MHRA’s IRP
- Approval was supported by 3 pivotal trials conducted by Akebia Therapeutics, which showed increased iron levels along with reduction in serum phosphorus in CKD pts
- Averoa licensed Xoanacyl from Akebia in Dec 2022, enhancing its dossier with a re-engineered clinical package to support dual indications for EU pts. Averoa is currently seeking commercial partners for EU launch & distribution
Company: Partner Therapeutics
Product: Imreplys
Active Ingredient: Sargramostim
Disease: Exposure to Myelosuppressive Doses of Radiation
Date: Jun 19, 2025
Shots:
- The CHMP has recommended Imreplys (sargramostim) to treat pts of all ages with Haematopoietic Sub-syndrome of Acute Radiation Syndrome (H-ARS) following myelosuppressive radiation exposure, across the EU, Norway, Iceland, and Liechtenstein
- Sargramostim is a recombinant human GM-CSF produced using yeast (S. cerevisiae) expression technology
- In 2018, the same formulation was approved by the US FDA as a brand name: Leukine (Partner Therapeutics)
Company: SpringWorks Therapeutics
Product: Ogsiveo
Active Ingredient: Nirogacestat
Disease: Desmoid Tumors
Date: Jun 19, 2025
Shots:
- The CHMP has recommended Nirogacestat as a monotx. for adults with progressing desmoid tumors needing systemic treatment, the EC’s decision is expected in Q3’25
- This opinion was based on P-III (DeFi) global trial (N=142) assessing Nirogacestat (n=700) vs PBO (n=72), which met the 1EP, showing a 71% reduction in PFS vs PBO, along with significant improvements in exploratory & 2EPs (ORR and PROs); published in the NEJM
- Nirogacestat (150mg; BID) is an oral, small-molecule gamma-secretase inhibitor approved in the US for adults with progressing desmoid tumors requiring systemic treatment
Company: Madrigal Pharmaceuticals
Product: Rezdiffra
Active Ingredient: Resmetirom
Disease: Non-cirrhotic MASH with mod. to advanced Liver Fibrosis
Date: Jun 19, 2025
Shots:
- The CHMP has recommended Rezdiffra (resmetirom) for the treatment of adults with noncirrhotic MASH with mod. to advanced liver fibrosis; the EC’s decision is expected by Aug 2025
- Opinion was based on P-III (MAESTRO-NASH) trial assessing Rezdiffra (100 & 80mg, PO, QD) vs PBO in MASH pts, which met its 1EPs of fibrosis improvement & MASH resolution, with 91% pts on Rezdiffra (100mg) showing improvement or stabilization in liver stiffness; data was published in The NEJM
- Rezdiffra is being evaluated in a fully enrolled, ongoing P-III (MAESTRO-NASH OUTCOMES) trial against PBO to assess its impact on liver decompensation in MASH cirrhosis
Company: ExCellThera
Product: Zemcelpro
Active Ingredient: Dorocubicel/Allogeneic umbilical cord-derived CD34- cells, non-expanded
Disease: Haematological Malignancies
Date: Jun 19, 2025
Shots:
- The CHMP has recommended conditional approval of Zemcelpro in 30 EEA states for adults with haematological malignancies requiring allogeneic HSCT after myeloablative conditioning with no suitable donor; EC’s decision is expected within 2mos.
- Zemcelpro (UM171 Cell Therapy) was evaluated in P-II trials for pts with high & very high-risk acute leukemias & myelodysplasias with limited therapy options & poor outcomes with SoCs, incl. those with TP53 mutations, refractory/active disease, or who require another transplant
- Zemcelpro will be advanced in a P-III trial for mentioned pts, plus additional filings are planned in multiple regulatory bodies incl. the US, Canada, the UK, & Switzerland
Company: PTC Therapeutics
Product: Sephience
Active Ingredient: Sepiapterin
Disease: Phenylketonuria
Date: Jun 23, 2025
Shots:
- The EC has approved Sephience (sepiapterin) to treat PKU in pts of all ages and disease severities across all 30 EEA states; launch is anticipated in Germany in H1 of July
- Approval was based on APHENITY study results and long-term extension data showing durable effects and improved dietary flexibility
- Sephience is an oral synthetic sepiapterin with a dual mechanism that boosts phenylalanine hydroxylase (PAH) enzyme activity. Sepiapterin’s NDA is under FDA review (PDUFA: July 29, 2025), and approval reviews are underway in countries like Japan and Brazil
Company: Jazz Pharmaceuticals
Product: Ziihera
Active Ingredient: Zanidatamab
Disease: Biliary Tract Cancer
Date: Jun 27, 2025
Shots:
- The EC has granted conditional approval to Ziihera in 30 EEA states for the treatment of inoperable locally advanced or metastatic HER2+ (IHC 3+) BTC adults, who were previously treated with ≥1L of therapy
- Approval was based on P-IIb (HERIZON-BTC-01) trial assessing Ziihera in 87 pts, where Arm 1 (n=80: 18 had IHC 2+ tumors & 62 had IHC 3+ tumors) achieved cORR (1EP) of 41.3% incl. 2 CR at mFU of 21.9mos., with mDoR of 14.9mos. & mOS of 15.5mos. In IHC 3+ tumor pts, cORR was 51.6%, with mDoR of 14.9mos. & mOS of 18.1mos.
- Ziihera’s continued approval depends on confirmation of clinical benefit in the ongoing P-III (HERIZON-BTC-302) trial assessing it with SoC vs SoC alone in 1L HER2+ BTC pts
Note: The following drug has been approved; however, no PR was available:
- Austedo (deutetrabenazine)
Related Post: Insights+: EMA Marketing Authorization of New Drugs in May 2025


