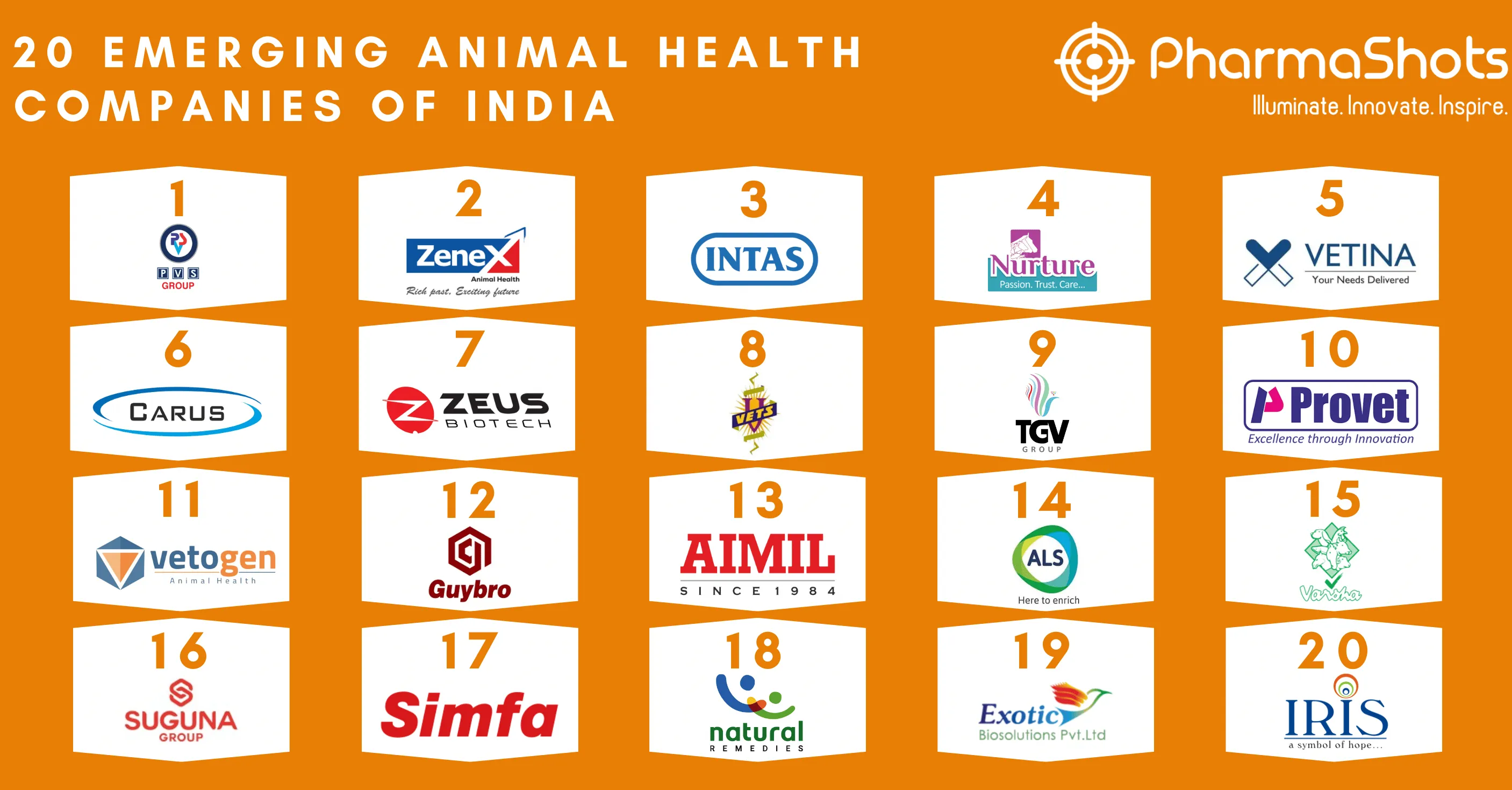
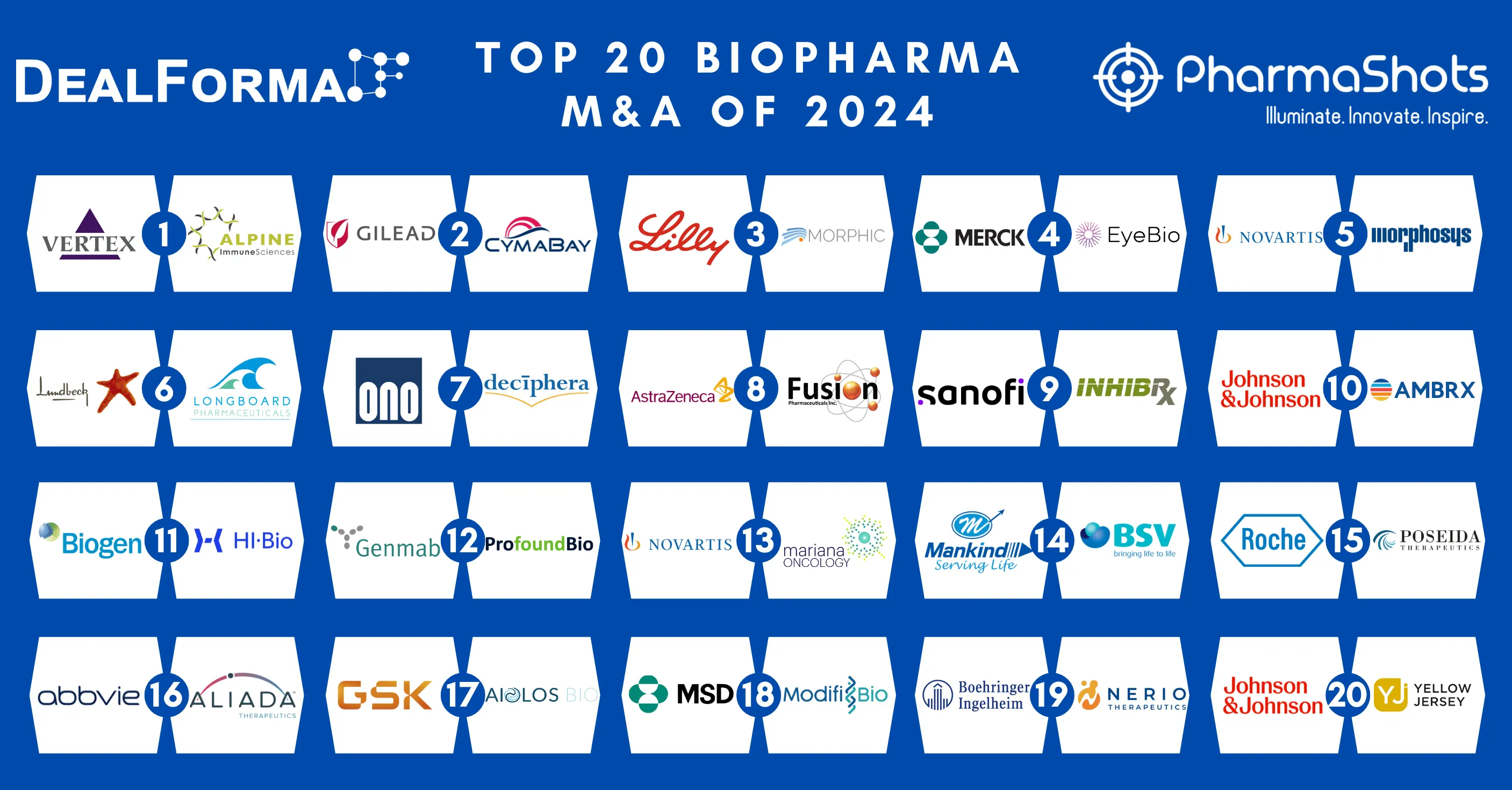
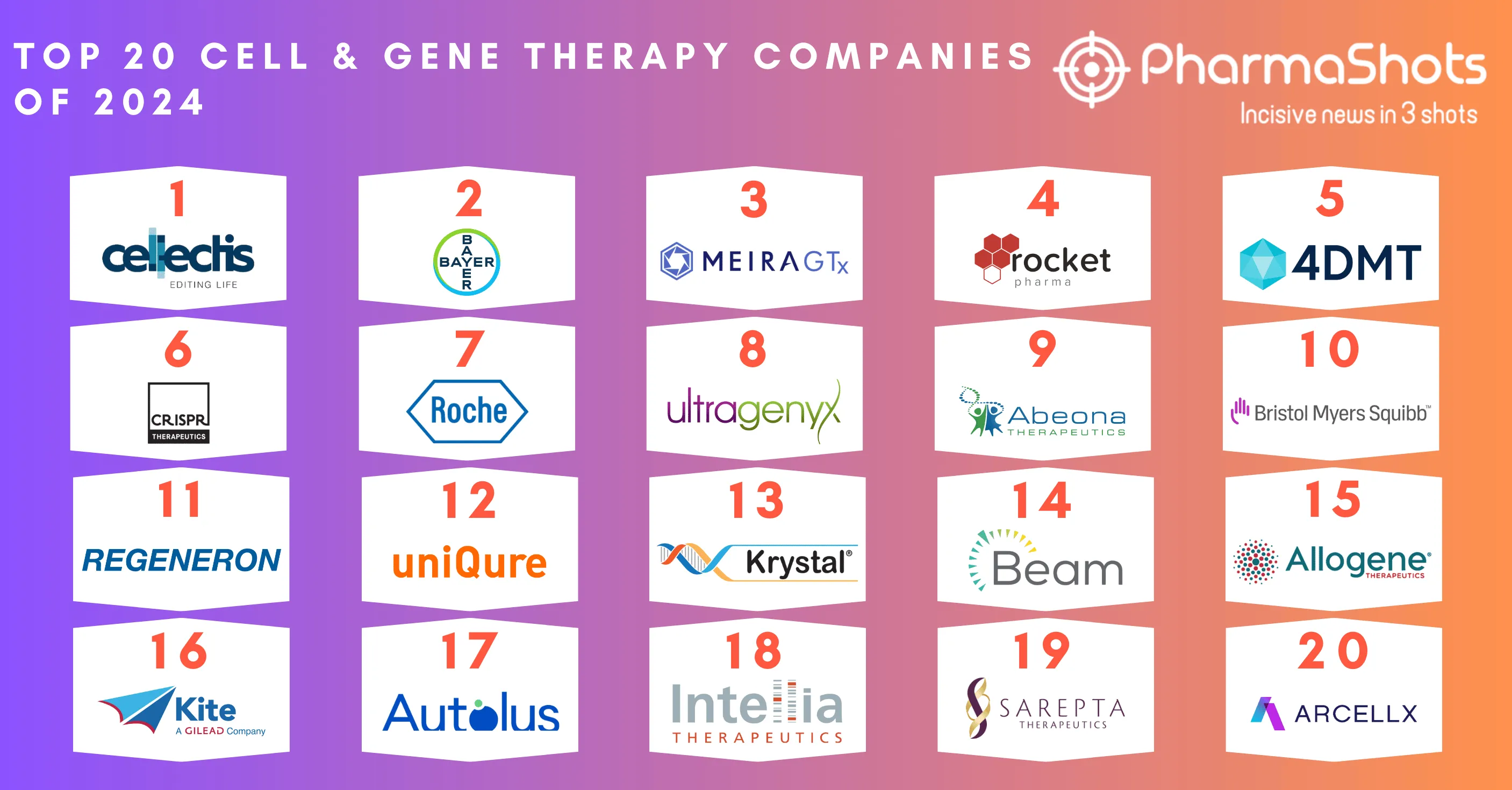
Top 20 Prescription Drugs Based on 2020 Revenue
- Innovative horizons are broadening continuously in the field of medicines. Health is not only limited to physical well-being, it also reflects the mental status
- Pharmaceutical medicines are advancing rapidly in every area of requirement such as immunology, nephrology, oncology, neurology, and Medicines that focuses on treating disease and improving health
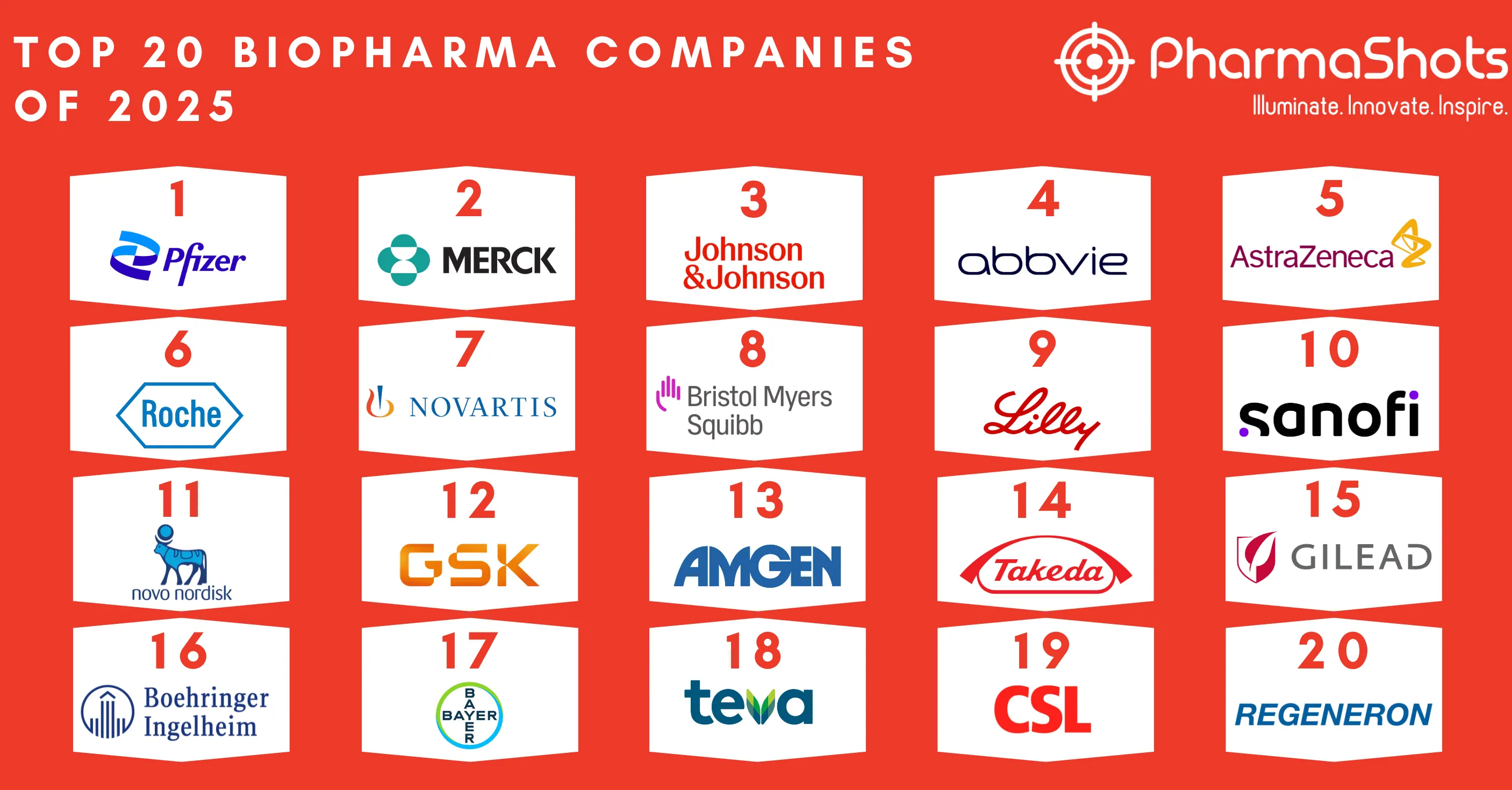
- We have compiled a list of global top 20 blockbuster prescription drugs based on their sales in 2020. In the Top 20 list, the top position was maintained by AbbVie's leading drug Humira with $20.35 of total sales in 2020

REMICADE
Product: Remicade
Company: Johnson & Johnson and Merck
First Approved: US (Aug 24, 1998), EU (Aug 13, 1999)
Total Revenue: $3.73B
Indications Approved: Crohn’s Disease, Rheumatoid Arthritis, Ankylosing Spondylitis, Plaque Psoriasis, Psoriatic Arthritis, Pediatric Ulcerative Colitis, and Pediatric Crohn’s Disease
Remicade is used for the treatment of a number of immune-mediated inflammatory diseases. In July 2021, Johnson & Johnson and Pfizer agreed to dismiss all claims asserted in the anti-trust suit brought by Pfizer in the Eastern District of Pennsylvania regarding Remicade. In 2020, J&J's leading drug, Remicade recorded total sales of $3.73B.

HERCEPTIN
Product: Herceptin
Company: Roche
First Approved: US (Sep 25, 1998), EU (Aug 28, 2000)
Total Revenue: $4.22B
Indications Approved: Metastatic Breast Cancer, Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma
Herceptin was the first HER2-targeted therapy for breast cancer. It is a monoclonal antibody that binds to HER2 receptors present on the surface of HER2-positive tumor cells, blocking them from receiving growth signals and flagging them for destruction by the immune system. Roche expects biosimilars to Herceptin, Avastin and Rituxan will cost the company roughly CHF 4.6 billion in 2021 sales. Sales of Herceptin were lower in 2020 in comparison to the previous year.

TAGRISSO
Product: Tagrisso
Company: AstraZeneca
First Approved: US (November 13, 2015), EU (April 25, 2017)
Total Revenue: $4.32B
Indications Approved: Non-Small Cell Lung Cancer
Tagrisso was the first approved medicine indicated for patients with metastatic EGFR T790M mutation-positive non-small cell lung cancer. In 2020, Tagrisso remained our top-selling medicine, as we continued its global rollout for 1st-line advanced EGFRm NSCLC and secured the first global approval in the adjuvant setting in the US in December 2020. In 2020, Oncology sales in Europe grew by 36% (35% at CER), driven by increased use of Tagrisso for the treatment of patients in the 1st-line EGFR7-mutated (EGFRm) non-small cell lung cancer (NSCLC) setting. In 2020, AstraZeneca’ Tagrisso recorded total sales of $4.32B.

RITUXAN
Product: Rituxan
Company: Roche
First Approved: US (Nov 26, 1997), EU (Jun 02, 1998)
Total Revenue: $4.77B
Indications Approved: Non-Hodgkin’s lymphoma, Chronic Lymphocytic Leukemia, Rheumatoid Arthritis, Granulomatosis with Polyangiitis, Microscopic Polyangiitis, and Pemphigus Vulgaris
Rituxan (rituximab) is a prescription medicine used to treat adults with Non-Hodgkin's Lymphoma (NHL). Rituxan is the first and only FDA-approved treatment for pediatric patients two years of age and older living with granulomatosis with polyangiitis or microscopic polyangiitis. In 2020, Rituxan recorded total sales of $4.77B. However, sales of Rituxan decreased in 2020 in comparison to that in 2019.

OCREVUS
Product: Ocrevus
Company: Roche
First Approved: US (March 28, 2017), EU (January 12, 2018)
Total Revenue: $4.89B
Indications Approved: Multiple Sclerosis
Ocrevus was the first and only approved disease-modifying medicine for people in the European Union (EU) with early primary progressive multiple sclerosis (PPMS). In 2020, sales of Ocrevus were much higher in comparison with the past year. In December 2020, FDA approved Ocrevus’ shorter two -hour infusion time for relapsing and primary progressive multiple sclerosis. In 2020, Ocrevus recorded total sales of $4.89B.

TRULICITY
Product: Trulicity
Company: Eli Lilly
First Approved: US (September 18, 2014), EU (November 25, 2014)
Total Revenue: $5.35B
Indications Approved: Type 2 Diabetes
Trulicity is used for the treatment of type 2 diabetes and to reduce the risk of major adverse cardiovascular events in adult patients with type 2 diabetes. In September 2020, FDA approved two additional doses of Trulicity. In 2021, revenue for Trulicity is expected to be driven by volume. In 2020, Eli Lilly leading drug, Trulicity recorded total sales of $5.35B.

IBRANCE
Product: Ibrance
Company: Pfizer
First Approved: US (Feb 03, 2015), EU (Nov 09, 2016)
Total Revenue: $5.39B
Indications Approved: HER2 Negative Advanced Breast Cancer
Ibrance is an oncology-category drug that is used in the treatment of metastatic breast cancer. Ibrance is approved in more than 100 countries. In 2020, Ibrance generated total sales of $5.39B. In 2020, the growth of Ibrance sales was largely driven compared to that in 2019.

AVASTIN
Product: Avastin
Company: Roche
First Approved: US (Feb 26, 2004), EU (Jan 12, 2005)
Total Revenue: $5.64B
Indications Approved: Metastatic Carcinoma, Metastatic Colorectal Cancer, non–Small Cell Lung Cancer, Glioblastoma, Metastatic Renal Cell Carcinoma, Metastatic Cervical Cancer, Primary Peritoneal Cancer, Hepatocellular Carcinoma
Avastin is an anti-cancer drug that interferes with the growth and spread of cancer cells in the body. Avastin is approved in over 130 countries worldwide. It is approved in the US for the treatment of colorectal and non-small cell lung cancer. Roche expects solid results from Avastin in 2021. In 2020, Avastin recorded total sales of $5.64B. However, the sales for Avastin largely decreased in 2020 I comparison to that in 2019.

PREVENAR 13
Product: Prevenar 13
Company: Pfizer
First Approved: US (Feb 24, 2010), EU (Dec 12, 2009)
Total Revenue: $5.85B
Indications Approved: S. Pneumoniae Infection
Prevnar 13 or Pneumococcal 13-valent Conjugate Vaccine is used in the treatment of pneumococcal pneumonia. In June 2020, FDA approved Prevnar 20, which is the first approval of a conjugate vaccine that helps protect against 20 serotypes responsible for the majority of invasive pneumococcal disease and pneumonia. In 2020, sales of Prevenar 13 increased with recorded revenue of $ 5.85B.

ENBREL
Product: Enbrel
Company: Amgen & Pfizer
First Approved: US (Nov 02, 1998), EU (Feb 02, 2000)
Total Revenue: $6.34B
Indications Approved: Rheumatoid Arthritis, Polyarticular-Course Juvenile Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Plaque Psoriasis
Enbrel is a tumor necrosis factor blocker that is used primarily in indications for the treatment of adult patients with moderately to severely active rheumatoid arthritis. In April 2019, FDA approved the second biosimilars version of Enbrel. In 2020, sales of Enbrel decreased in comparison to that recorded in 2019.

IMBRUVICA
Product: Imbruvica
Company: Johnson & Johnson and AbbVie
First Approved: US (Nov 13, 2013), EU (Nov 21, 2014)
Total Revenue: $6.61B
Indications Approved: Mantle Cell Lymphoma, Chronic Lymphocytic Leukemia
Imbruvica is used in the treatment of certain B-cell malignancies. In June 2021, J&J announced that Imbruvica along with Venclexta helped elderly cancer patients live longer without disease progression in a late-stage trial compared with chemoimmunotherapy. Imbruvica is the company’s third-largest product that accounting for approximately 5.0% of the company's total revenue for fiscal 2020. In 2020, Imbruvica recorded total sales of $6.61B.

BIKTARVY
Product: Biktarvy
Company: Gilead Sciences
First Approved: US (February 7, 2018), EU (June 25, 2018)
Total Revenue: $7.25B
Indications Approved: HIV-1 infection
Biktarvy is a single tablet regimen (STR) that is used in the treatment of HIV-1 infection. In October 2020, USFDA approved Expanded Indication for Biktarvy for the treatment of HIV-1 infection in pediatric populations. In 2020, Gilead’ leading drug Biktarvy recorded total sales of $7.25B.

STELARA
Product: Stelara
Company: Johnson & Johnson
First Approved: US (Sep 25, 2009), EU (Jan 15, 2009)
Total Revenue: $7.70B
Indications Approved: Plaque Psoriasis, Psoriatic Arthritis, Crohn’s Disease, and Ulcerative Colitis
Stelara or ustekinumab is used in the treatment of adults and children with moderate to severe plaque psoriasis, adults with active psoriatic arthritis, and adults with moderately to severely active Crohn’s disease and treatment of moderately to severely active ulcerative colitis. Stelara is Johnson and Johnson's largest selling product that accounting for approximately 9.3 percent of the company’s total revenue in 2020. In June 2021, the Janssen Pharmaceutical Companies of Johnson & Johnson reported five-year data from the Phase III IM-UNITI trial of Stelara (ustekinumab) that showed long-term remission in patients with moderate to severe Crohn's disease (CD). In January 2020, the European Commission approved the expanded use of Stelara for the treatment of pediatric patients with moderate to severe plaque psoriasis. In 2020, Stelara recorded total sales of $7.70B.

OPDIVO
Product: Opdivo
Company: BMS and Ono Pharmaceuticals
First Approved: US (Dec 22, 2014), EU (Jun 19, 2015)
Total Revenue: $7.84B
Indications Approved: Metastatic Melanoma, Non-Small Cell Lung Cancer, Melanoma, Small Cell Lung Cancer, Renal Cell Carcinoma, Hodgkin Lymphoma, Squamous Cell Carcinoma of the Head and Neck, Urothelial Carcinoma, Mismatch Repair Deficient Metastatic Colorectal Cancer, Hepatocellular Carcinoma, Esophageal Squamous Cell Carcinoma
Opdivo or nivolumab is a prescription medicine that is used in the treatment of certain types of cancers including non-small cell lung cancer and colorectal cancer. Opdivo based treatment shows promising outcomes in cancer patients. In January 2021, the European Medicines Agency validated BMS’ Marketing Authorization Application for Opdivo as an adjuvant treatment for esophageal or gastroesophageal junction cancer in adult patients with residual pathologic disease after neoadjuvant chemoradiotherapy and resection. In 2020, BMS’ Opdivo generated total revenue of $7.84B.

XARELTO
Product: Xarelto
Company: Johnson & Johnson and Bayer
First Approved: US (Nov 4, 2011), EU (Sep 30, 2008)
Total Revenue: $7.86B
Indications Approved: blood clots, coronary artery disease, and peripheral artery disease
Xarelto (rivaroxaban) is an oral anticoagulant for the prevention of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE) in patients undergoing hip or knee replacement surgery, to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, and for the treatment and reduction of risk of recurrence of DVT and PE. In Oct 2020, Janssen submitted an application to US FDA for a new indication to expand the use of Xarelto in patients with peripheral artery disease. In 2020, Xarelto recorded total revenue of $7.86B.

EYLEA
Product: Eylea
Company: Regeneron Pharmaceuticals & Bayer
First Approved: US (Nov 18, 2011), EU (Nov 21, 2012)
Total Revenue: $7.96B
Indications Approved: Age-Related Macular Degeneration, Macular Edema, Diabetic Macular Edema, Diabetic Retinopathy
Eylea or aflibercept is a prescription medicine that is used in the treatment of neovascular age-related macular degeneration (wet AMD), diabetic macular edema (DME), macular edema following retinal vein occlusion (RVO), which includes macular edema following central retinal vein occlusion (CRVO) and macular edema following branch retinal vein occlusion (BRVO). EYLEA is available in Japan and the EU for the treatment of myopic choroidal neovascularization (mCNV) and in the United States for the treatment of diabetic retinopathy in patients with DME. In February 2020, Regeneron announced positive results of the Phase 3 PANORAMA Trial evaluating Eylea in patients with moderately severe to severe non-proliferative diabetic retinopathy (NPDR). In 2020, Eylea generated total sales of $7.96B.

REVLIMID
Product: Revlimid
Company: BMS and BeiGene
First Approved: US (Dec 27, 2005), EU (Jun 14, 2007)
Total Revenue: $12.14B
Indications Approved: Anemia, Multiple Myeloma, Myelodysplastic Syndromes, Mantle Cell Lymphoma, Follicular Lymphoma, Marginal Zone Lymphoma.
Revlimid (lenalidomide) is an oral immunomodulatory drug that in combination with dexamethasone is indicated for the treatment of patients with multiple myeloma. In November 2020, BMS announced the settlement of US Patent Litigation for Revlimid with Cipla. In 2020, BMS’ leading drug, Revlimid recorded total sales of $12.14B.

ELIQUIS
Product: Eliquis
Company: BMS and Pfizer
First Approved: US (Dec 28, 2012), EU (May 18, 2011)
Total Revenue: $13.89B
Indications Approved: Stroke, Systemic Embolism, Deep Vein Thrombosis, Pulmonary Embolism, Recurrence of DVT and PE
Eliquis or apixaban is an oral Factor Xa inhibitor indicated for the reduction in risk of stroke/systemic embolism in NVAF and for the treatment of DVT/PE and reduction in risk of recurrence following initial therapy. BMS and Pfizer jointly develop and commercialize Eliquis. In 2020, Eliquis showed higher demand in US market and generated total revenue of $13.89B.

KEYTRUDA
Product: Keytruda
Company: Merck and Co.
First Approved: US (Sep 04, 2014), EU (Jul 17, 2015)
Total Revenue: $14.38B
Indications Approved: Metastatic Melanoma, Non-Small Cell Lung Cancer, Small Cell Lung Cancer, Head, and Neck Squamous Cell Cancer, Hodgkin Lymphoma, Mediastinal Large B-Cell Lymphoma, Urothelial Carcinoma, Deficient Cancer, Colorectal Cancer, Gastric Cancer, Esophageal Cancer, Cervical Cancer, Hepatocellular Carcinoma, Merkel Cell Carcinoma, Renal Cell Carcinoma, Endometrial Carcinoma, Tumor Mutational Burden-High Cancer, Cutaneous Squamous Cell Carcinoma
Keytruda is an oncology drug that is used in the treatment of advanced melanoma and metastatic non-small-cell lung cancer (NSCLC) in patients whose tumors express PD-L1. In October 2021, Merck announced that the U.S. Food and Drug Administration (FDA) approved Keytruda for the treatment of patients with persistent, recurrent, or metastatic cervical cancer whose tumors express PD-L1. In January 2020, FDA approved Keytruda for patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle-invasive bladder cancer with carcinoma in situ with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy. In 2020, Merck's key drug, Keytruda recorded total revenue of $14.38B.

HUMIRA
Product: Humira
Company: AbbVie
First Approved: US (Dec 31, 2002), EU (Aug 09, 2003)
Total Revenue: $20.35B
Indications Approved: Rheumatoid Arthritis, Juvenile Idiopathic Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Crohn’s Disease, Ulcerative Colitis, Plaque Psoriasis, Hidradenitis Suppurativa, and Uveitis
Humira (adalimumab) is a biologic therapy administered as a subcutaneous injection. Humira is approved for the treatment of Rheumatoid arthritis (moderate to severe), Psoriatic arthritis, Ankylosing spondylitis, Adult Crohn’s disease (moderate to severe), Plaque psoriasis (moderate to severe chronic) in North America and the European Union. Humira is also approved in Japan for the treatment of pyoderma gangrenosum. In 2020, Abbvie's leading drug, Humira accounted for approximately 43 percent of Abbvie’s total net revenues. In 2020, Humira recorded high demand sales of $20.35B.
Related Post: Top 20 Prescription Drugs Based on 2019 Revenue

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com