
ILAP and its nuance vs PRIME since BREXIT
Introduction
PRIME (PRIority MEdicines)
EMA launched PRIME on March 07, 2016, to support the development of medicines targeting unmet medical needs. The scheme is based on enhanced interaction and early dialogue with developers of promising medicines, to optimize development plans and speed up evaluation so these medicines can reach patients faster.
PRIME builds on the existing regulatory framework and tools already available such as scientific advice and accelerated assessment. Drug developers that benefitted from PRIME can expect to be eligible for accelerated assessment at the time of application for marketing authorization.1
ILAP
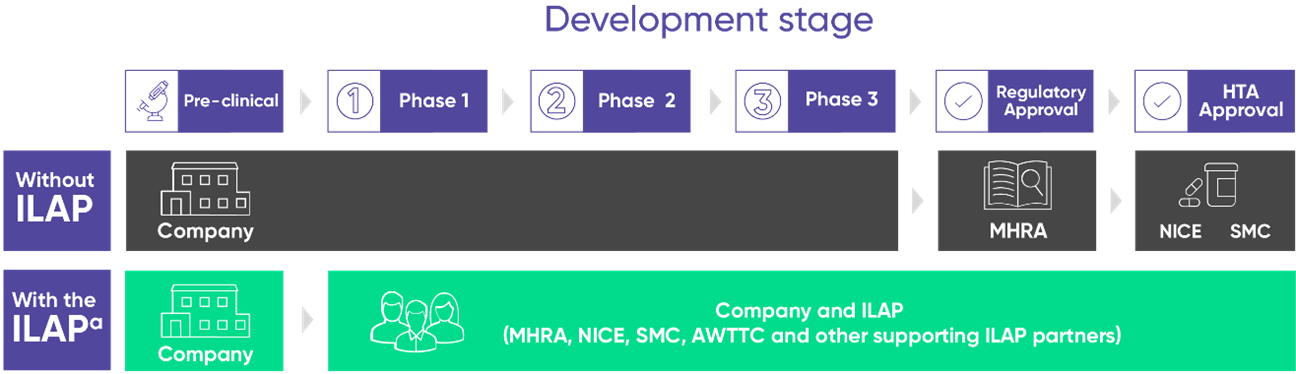
Since the UK moved out of Europe on January 31, 2020, the Medicines and Healthcare products Regulatory Agency (MHRA) became the sole body for the regulation of medicines in the UK. Post Brexit, a new and unprecedented era was born for the approval of medicines in the UK.
The ILAP was launched on January 01, 2021, as a pioneering pathway for commercial and non-commercial product developers. ILAP is a single integrated platform for collaborative consultation with both regulatory & reimbursement bodies. For ILAP, MHRA, NICE, SMC, and AWTTC had come together with supporting partners (NHS England and NHS Improvement, HRA, NIHR)3 to facilitate a unified process for streamlining the “bench to bedside” pathway.2
Comparison of UK market access route with and without the ILAP2
Eligibility Criteria
For PRIME
The eligibility criteria for PRIME are identical to EMA’s accelerated assessment criteria, and target medicinal products of major public health interest and in particular from the viewpoint of therapeutic innovation.
Products eligible for PRIME support shall:
- Target conditions where there is an unmet medical need, i.e. for which there exists no satisfactory method of diagnosis, prevention, or treatment in the Community or, even if such a method exists, in relation to which the medicinal product concerned will be of major therapeutic advantage to those affected;
- Demonstrate the potential to address the unmet medical need for maintaining and improving the health of the Community, for example, by introducing new methods of therapy or improving existing ones.4
For ILAP
Criteria 1: Details of the condition, patient, or public health area3
- life-threatening or seriously debilitating condition
- significant patient or public health need for the new product
Criteria 2: Medicinal product fulfilled one or more areas3
- An innovative medicine such as an advanced therapy medicinal product (ATMP) or new chemical or biological entity or novel drug-device combination
- A medicine being developed in a clinically significant new indication for an approved medicine
- Rare disease and/or other special populations such as neonates and children, elderly and pregnant women
- Development aligns with the objectives for UK public health priorities such as the Chief Medical Officer, Department of Health and Social Care (DHSC), or Life Sciences Sector Deal (including those in Devolved Administrations, where appropriate)
Criteria 3: Medicinal product has the potential to offer benefits to patients3
Fees

*TDP=Target Development Profile
Regulatory Update
PRIME
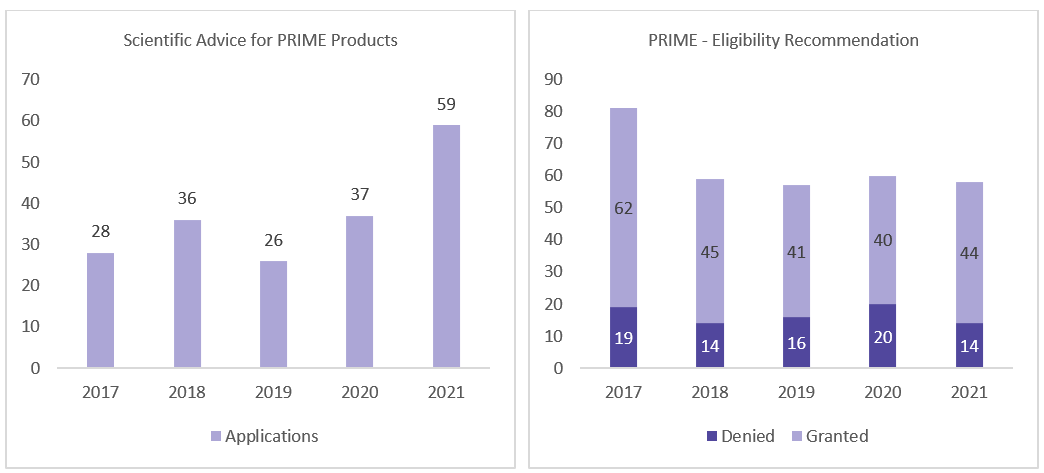
EMA reported that its PRIME scheme for priority medicines has effectively cut the time to approval for the products that qualify to use the scheme, but it is already planning how to update the program to target the assistance more precisely.
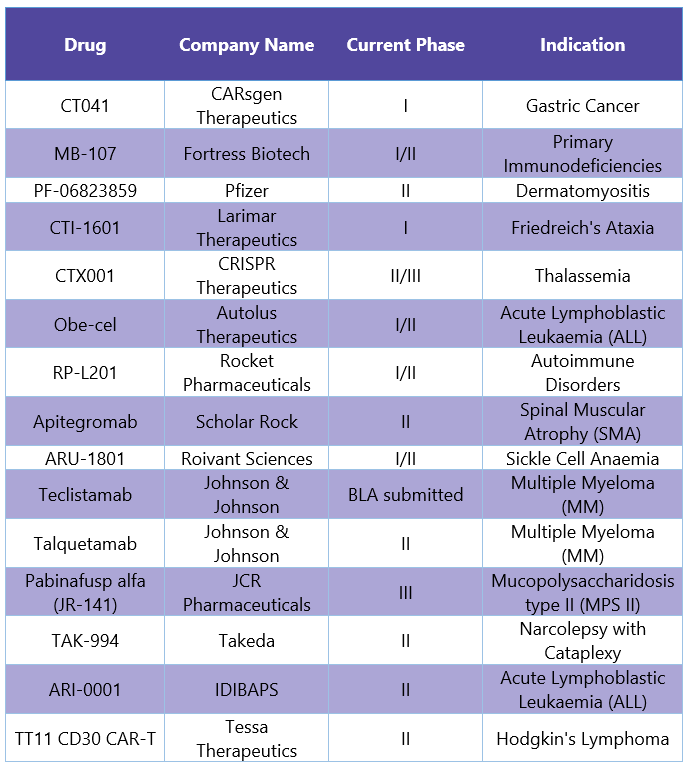
The first 5 years of PRIME designation enabled "earlier availability of life-changing medicines’’. As of March 2022, 98 medicines have qualified for the scheme, including CAR-T cell therapies, curative gene therapies, treatments for rare cancers, and a vaccine to protect against the Ebola virus.5
From Mar 2016 to Jun 2021, 18 medicines that had PRIME support obtained EU approval, 10 of them with a CMA. Of the approved products, 7 are advanced therapies and had on average shorter active assessment time and clock-stop duration than the average assessment time for all types of new active substances in 2020. 16 treatments are for rare diseases.5
In the year 2021, EMA granted PRIME designation to 12 therapies, most of them in P-I and P-II. Out of 12, 5 targeted oncology indications, and all of them either have been granted with Breakthrough Designation, Fast Track designation, or Orphan Designation.
PharmaShots has listed all the drugs that received PRIME designation in the former year1.
 ILAP
ILAP
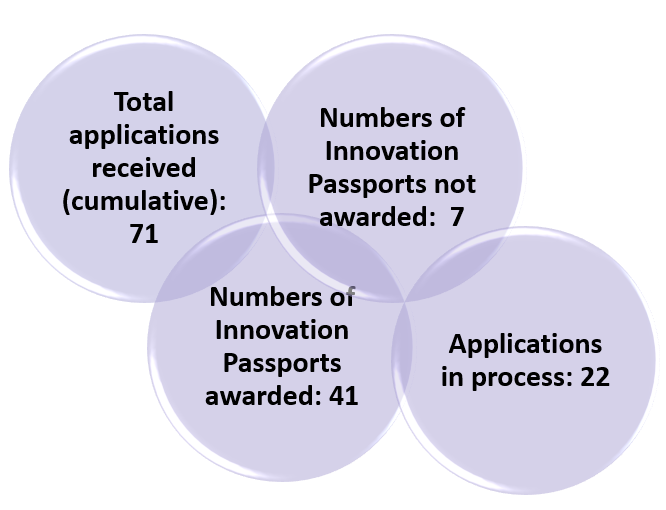
The year 2021 is UK’s ILAP first year of operations and a total of 71 applications were received last year from companies to take advantage of the regulatory advice, tools and flexibilities on offer.
The MHRA (UK’s regulatory body) awarded a total of 41 “innovation passports” for new drugs under development last year. The grant of a passport opens the door to the agency’s Innovative Licensing and Access Pathway (ILAP), which was introduced in January 2021.
MHRA unveiled that out of 71 received applications, seven were not granted and 22 applications are in process by the end of the year.3
Applications received have included new and established medicinal products in both common and rare diseases in a variety of therapeutic areas (the top three areas of interest currently are oncology, neurology and respiratory). Applications have been received from a range of companies in the pharmaceutical industry, including small and mid-size enterprises.
Receiving a passport means that the drug’s sponsor can begin putting together a “target development profile” (TDP), which is essentially a “roadmap” that will define the product’s key regulatory and development features and identify potential pitfalls. The TDP is intended as a “living document” that is updated as the development program proceeds.6
The agency also reveals that the products entering the ILAP were at very different stages of development, although it stressed that joining the pathway at an early stage was likely to be more beneficial in terms of building the TDP and using the available development tools.
On February 26, 2021, the agency awarded its first Innovation Passport to MSD’s Belzutifan for adults with von Hippel Lindau disease (a rare genetic disorder that causes cancer). This means patients could benefit much sooner from this treatment and it will be accelerated through the approval process; the Innovative Licensing and Access Pathway (ILAP).7
References:
- https://www.ema.europa.eu/en/human-regulatory/research-development/prime-priority-medicines
- https://www.xcenda.com/insights/htaq-spring-2022-uk-innovative-licensing-and-access-pathway
- https://www.gov.uk/guidance/innovative-licensing-and-access-pathway
- https://www.ema.europa.eu/en/documents/other/european-medicines-agency-guidance-applicants-seeking-access-prime-scheme_en.pdf
- https://www.appliedclinicaltrialsonline.com/view/ema-product-prioritization-can-cut-approval-delays-but-could-be-better-targeted
- https://pink.pharmaintelligence.informa.com/PS145471/UK-Regulator-Issues-41-Innovation-Passports-In-First-Year-Of-New-Pathway
- https://www.gov.uk/government/news/first-innovation-passport-awarded-to-help-support-development-and-access-to-cutting-edge-medicines
- https://www.ema.europa.eu/en/documents/annual-report/2021-annual-report-european-medicines-agency_en.pdf
Related Post: The Emergence of Artificial Intelligence in the Pharmaceutical Industry
Tags

Senior Editor at PharmaShots. She is curious and very passionate about recent updates and developments in the life sciences industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots.












