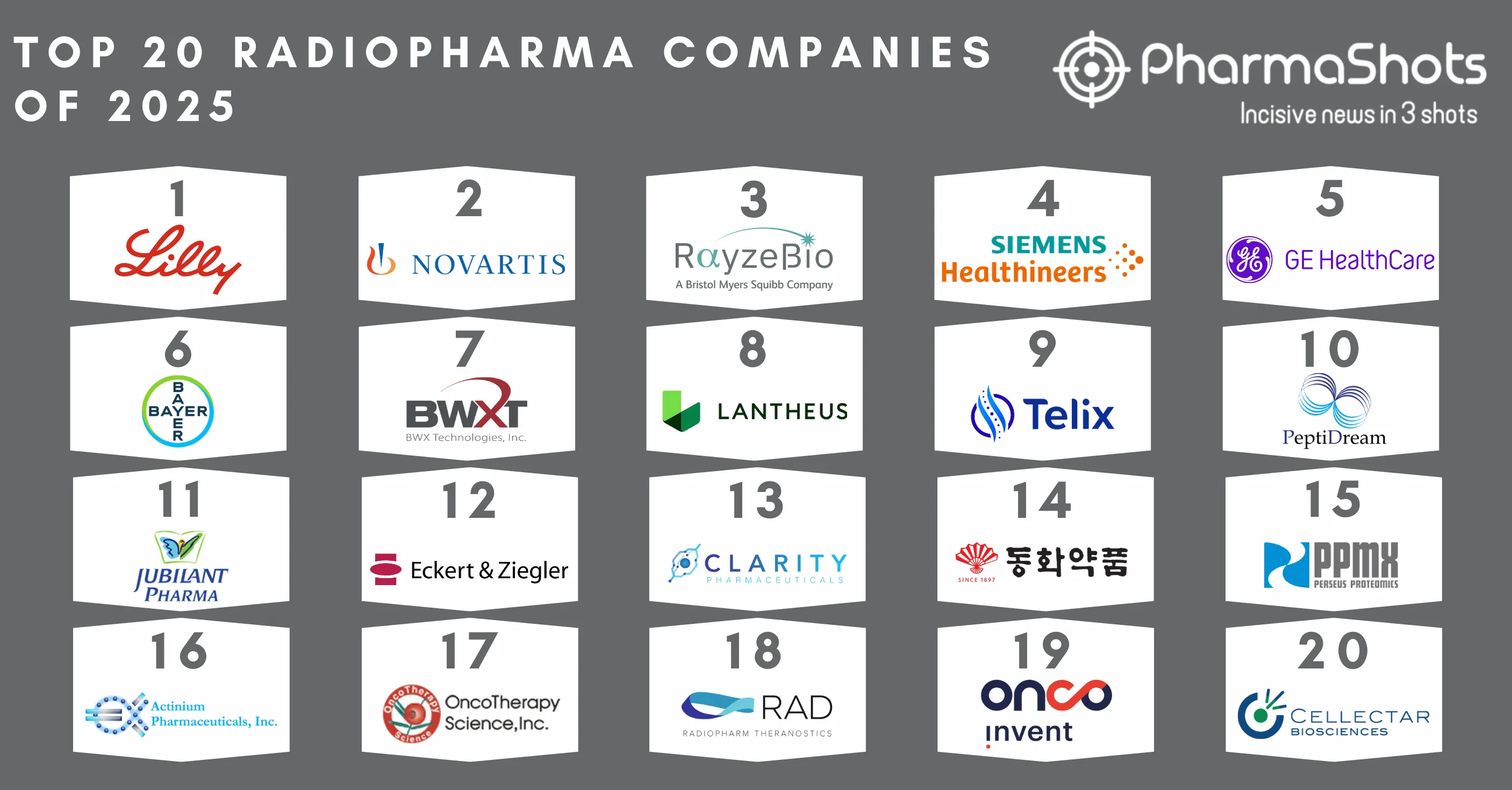
Top 20 Generics Pharma Companies Based On 2018 Revenue
The global generics pharmaceutical market is continuously growing and increasing with its accessibility & benefits offered as efficient and economical drugs- are always needed in the market. Generics are approved copies of small molecule drugs which contain the same amount of active ingredients- dosage form- safety- strength- route of administration- quality- performance characteristics- as of originally marketed products. In the list of top 20 companies- Mylan retained the top position with a revenue of $11.43B while there was a 4% dip in its revenue as compared to 2017. Our team at PharmaShots has compiled a list of top 20 generics developing companies based on their 2018 generics revenue.

Generic Segment Revenue: $0.69B Total Employees: ~7820
Founded Year: 1980 Headquarter: Mumbai- India
Market Cap: ~$5.18B Stock Exchange: NSE
Piramal Healthcare is a global pharmaceutical company offering therapies focussed on CNS- cardiovascular- anti-infective and anti-diabetic. In 2018- Primal launched its generic Desflurane as an inhalation vapour anaesthesia. Additionally- Piramal was granted 781 ANDAs approval in 2018 from the US FDA.

Generic Segment Revenue: $0.90B Total Employees: ~3700
Founded Year: 1867 Headquarter: Surrey- United Kingdom
Market Cap: ~$2.88B Stock Exchange: NYSE
Mallinckrodt is a global pharmaceutical company that develops and manufactures therapies for multiple therapy areas including autoimmune and rare diseases in speciality areas like neurology- rheumatology- nephrology- pulmonology and ophthalmology- immunotherapy and neonatal respiratory critical care therapies- and analgesics.

Generic Segment Revenue: $1.01B Total Employees: ~2-910
Founded Year: 1997 Headquarter: Dublin- Ireland
Market Cap: ~$1.00B Stock Exchange: NASDAQ
Endo International is an Ireland-based- global pharmaceutical company focusing on generic and branded pharmaceuticals for therapeutic areas that include Endocrinology- Medical Aesthetics- Orthopedics- Urology & Men's Health. Endo's Percocet (Oxycodone/acetaminophen) is an opioid analgesic approved for the treatment of moderate-to-moderately-severe pain and has generated global sales of $0.12B.

Generic Segment Revenue: $1.11B Total Employees: ~13-598
Founded Year: 1959 Headquarter: Ahmedabad- India
Market Cap: ~$4.05B Stock Exchange: NSE
Torrent Pharma is a pharmaceutical company focused on developing therapies for Cardiovascular- Central Nervous System- GI- Vitamins Minerals Nutritionals (VMN) and Women Healthcare. The Company also has a focus in diabetology- pain management- gynaecology- oncology and anti-infective segments. In Jan 2018- the company acquired Bio-Pharm for the expansion of its capabilities in the US including manufacturing and R&D presence.

Generic Segment Revenue: $1.43B Total Employees: ~12-000
Founded Year: 1977 Headquarter: Mumbai- India
Market Cap: ~$1.47B Stock Exchange: NSE
Glenmark is a global pharmaceutical company focusing on Generics- Specialty and OTC business in therapy areas like Dermatology- Respiratory and Oncology including Cardiology- Diabetes- and Oral Contraceptives. In 2018- the company received 25 ANDA approvals with 20 final approvals and 5 tentative approvals. Glenmark's Colesevelam Hydrochloride Tablets is first generic available as an oral suspension. Glenmark's generic portfolio consists of 152 generic products approved for marketing in the US.

Generic Segment Revenue: $1.66B Total Employees: ~6-000
Founded Year: 2002 Headquarter: New Jersey- United States
Market Cap: ~$1.04B Stock Exchange: NYSE
Amneal Pharmaceuticals is a global company developing and marketing generic and specialty drugs in multiple dosage forms for various diseases. The generic drug has generated a global sale of $0.13B with 8% increment to 2018. In Aug 2018 Amneal signed a 10-year license and supply agreement with Jerome Stevens for its Levothyroxine sodium tablets and will be effective in H1'19.

Generic Segment Revenue: $1.55B Total Employees: ~10-416
Founded Year: 1895 Headquarter: Bad Vilbel- Germany
Market Cap: $5.74B Stock Exchange: Xetra
STADA is an international pharmaceutical company which focuses on Generics and Branded Products. Its branded portfolio includes non-prescription (OTC)- prescription (RX) and discretionary prescription (OTX) products. Naxolone (Tilidin Naloxone) is a generic drug targeted for pain and has generated worldwide sales of $0.42B with an increment of 4% to 2018. In 2018- their generic segment contributed 59% of the sales.

Generic Segment Revenue: $1.69B Total Employees: ~100-000
Founded Year: 1973 Headquarter: Paris- France
Market Cap: ~$111.10B Stock Exchange: Euronext
Sanofi is a leading global healthcare company- focused on patient needs and engaged in the research- development- manufacturing and marketing of therapeutic solutions. The company is focused on Rare Diseases- Multiple Sclerosis- Immunology- Rare Blood Disorder- Oncology- Diabetes- Cardiovascular- Established Prescription Products- Generics- Consumer Healthcare- and Vaccines. In Sept 2018- Sanofi completed the divestment of their European generics business Zentiva to Advent International. Additionally- Sanofi launched authorized generics of Renvela (sevelamer carbonate) in the US market as tablet and powder formulation in 2017 and 2018 respectively

Generic Segment Revenue: $1.70B Total Employees: ~3-131
Founded Year: 1929 Headquarter: Osaka- Japan
Market Cap: ~$2.32B Stock Exchange: TOKYO
Sawai Pharmaceutical is a generic pharmaceutical focus on drugs for multiple therapy areas i.e. cardiovascular- antihyperlipidemic agents- diabetes drugs- anticancer drugs- and OTC drugs. In Dec 2018 Sawai launched six generic products with 14 strengths namely Mirtazapine OD Tablets 15/30 mg- Atomoxetine Capsules 5/10/25/40 mg- Toaraset- Ropinirole ER Tablets 2/8 mg- Frewell- Capecitabine 300 mg. Additionally- the company retains 310 products with 756 strengths.

Generic Segment Revenue: $1.73B Total Employees: ~26000
Founded Year: 1935 Headquarter: Mumbai- India
Market Cap: ~$5.29B Stock Exchange: NSE
Cipla is a global leading pharmaceutical company ranked at 10th position among the US firms. Cipla is a leading firm in generics that majorly focusses on Cardiology- Oncology and Diabetology. Cipla's Isuprel (isoproterenol hydrochloride) is a generic drug administered as IV- IM- SC and intracardiac routes used for the treatment of bradycardia- heart block- and rarely for asthma. In 2018- the company has launched 15 generic products and filed 15 generic in-house filings with two in-licensing products.

Generic Segment Revenue: $1.77B Total Employees: ~21-966
Founded Year: 1984 Headquarter: Telangana- India
Market Cap: ~$6.32B Stock Exchange: NSE
Dr Reddy's Laborataries is a global pharmaceutical company with three divided segments Pharmaceutical Services & Active Ingredients- Global Generics and Proprietary Products. The company focusses on GI- cardiovascular- diabetology- oncology- pain management and anti-infective. In 2018- the company filed 20 ANDAs with the US FDA along with 110 generic filings pending approval- comprising 107 ANDAs and 3 NDA filed under the Section 505(b)(2) route.

Generic Segment Revenue: $2.01B Total Employees: ~8-400
Founded Year: 1978 Headquarter: London- United Kingdom
Market Cap: ~$5.80B Stock Exchange: LSE
Hikma is a global pharmaceutical firm focusing on the development and marketing of generics- branded products and injectables anti-infectives- cardiovascular- CNS- diabetes including oncology- pain management and respiratory. The company's generic segmnet has demonstrated 13% increment to 2017. The company launched 90+ products in 2018. Additionally- in 2018 the company received the US FDA?s approval for Ritonavir Tablets USP- 100 mg used in combination with antiretroviral agents for treatment of human immunodeficiency virus (HIV-1) infection.

Generic Segment Revenue: $2.25B Total Employees: ~20-000
Founded Year: 1968 Headquarter: Mumbai- India
Market Cap: ~$4.82B Stock Exchange: NSE
Lupin is a pharmaceutical firm involved in developing and delivering a wide range of generic formulations- biosimilar products and APIs globally. The company holds 3rd largest position as pharmaceutical-based on global revenue in India and 6th as a generic leader in Japan. The company is focused on multiple therapy area including Cardiovascular- Diabetology- Asthma- Pediatric- CNS- GI- Anti-Infective and NSAID space and holds a global leadership position for the Anti-TB segment.
Generic Segment Revenue: $2.83B Total Employees: ~22000
Founded Year: 1986 Headquarter: Telangana- India
Market Cap: ~$5.14B Stock Exchange: NSE
Aurobindo Pharma Limited is a world-leading manufacturer of generic pharmaceuticals and active pharmaceutical ingredients. The company is focused on therapeutic/product areas encompassing Antibiotics- Antiretrovirals- CVS- CNS- Systemic Gastroenterologicals- Anti-Allergies- Anti-Diabetics and other therapeutic areas. In 2018- Aurobindo acquires Apotex Business in Poland- Czech Republic- the Netherlands- Spain and Belgium to strengthen its approach and capabilities of generic products in European markets.

Generic Segment Revenue: $3.04B Total Employees: ~112-658
Founded Year: 1996 Headquarter: Bad Homburg- Germany
Market Cap: ~25.03B Stock Exchange: Xetra
Fresenius Kabi is a global healthcare company that develops and provide products as well as services for Dialysis- Generic & IV drugs- Biosimilars- Medical Devices in addition to Infusion and Nutrition therapies. Fresenius portfolio has around has around 90+ intravenously administered generic anaesthetics, analgesics, anti-infectives and drugs treat multiple disorders. In 2018, the company has generated a total rise of 5% in its generic sales.
.png)
Generic Segment Revenue: $4.10B Total Employees: ~32,000
Founded Year: 1983 Headquarter: Mumbai, India
Market Cap: ~$14.37B Stock Exchange: BSE
Sun Pharma is the world's fourth-largest and India's top generic pharmaceutical company. In 2018, the company launched its generic version of Glumetza Tablets (500 mg, 1000mg) extended-release tablets in the US. In Aug 2018, the company also received approval for Cequa (cyclosporine ophthalmic solution, 0.09%) from the US FDA. Sun Pharma has a pipeline of 118 ANDAs and 8 NDAs pending with the US FDA.
.png)
Generic Segment Revenue: $5.2B Total Employees: ~92,400
Founded Year: 1849 Headquarter: New York, United States
Market Cap: ~$206.30B Stock Exchange: NYSE
Pfizer is a research-based global biopharmaceutical company with a portfolio of Internal Medicine, Vaccines, Oncology, Inflammation & Immunology, Rare Disease and Consumer Healthcare.
Upjohn-Pfizer's generic business unit has off-patent branded and generic products business, headquartered in China that includes 20 off-patent solid oral dose brands as well as certain generic products. In 2018, Pfizer’s generic Sulperazon (sulbactam sodium/cefoperazone sodium) targeted for urinary tract infection, has generated a global sales revenue of $ 0.61B.
.png)
Generic Segment Revenue: $8.42B Total Employees: ~15,000
Founded Year: 1886 Headquarter: Holzkirchen, Germany
Market Cap: ~$46B Stock Exchange: NA
Sandoz, a Novartis division is a global leader in developing, manufacturing and marketing finished dosage form medicines as well as intermediary products including active pharmaceutical ingredients. The company is organized globally into three divisions Retail Generics, Anti-Infectives and Biopharmaceuticals. Sandoz is focused on therapies for Cardiovascular, CNS, Dermatology, Gastrointestinal & Hormonal disorders, Metabolism, Oncology, Ophthalmic pain and Respiratory disorders. Additionally, in 2018 Novartis agreed to sell a selected portion of the US portfolio specifically the Sandoz US dermatology business and generic US oral solids portfolio to Aurobindo Pharma.
.png)
Generic Segment Revenue: $9.67B Total Employees: ~42,535
Founded Year: 1944 Headquarter: Petah Tikva, Israel
Market Cap: ~$31.77B Stock Exchange: NYSE
Teva is a global pharmaceutical firm leading in generic products with a portfolio consisting of over 35,000 products in every therapy area. The products manufactured include a variety of dosage forms, including tablets, capsules, injectables, inhalants, liquids, ointments and creams. In 2018, Teva's generic Copaxone (glatiramer acetate, IV) has generated sales of $2.36B used to treat relapsing forms of multiple sclerosis (MS), relapsing-remitting disease, and active secondary progressive disease, in adults.
.png)
Generic Segment Revenue: $11.43B Total Employees: ~35,000
Founded Year: 1961 Headquarter: Pennsylvania, United States
Market Cap: ~$11.47B Stock Exchange: NASDAQ
Mylan is a global pharmaceutical company focused on manufacturing of prescription generic, branded generic, brand-name including biosimilar drugs and OTC drugs for multiple diseases. The generics segment has demonstrated 4% decrement as compared to 2017. The company launched more than ten complex generics and biosimilar products in 2018 such as Bivalirudin (generic Angiomax), Tadalafil (generic Adcirca), Dalfampridine (generic Ampyra), and Mesalamine Rectal Suppository (generic of Allergan’s Canasa Rectal Suppository). Recently, in July 2019, Pfizer announced to merge its Upjohn off-patent drug business with Mylan to form a new pharmaceutical company.
Related Post: Top 20 Life Sciences Deals of 2018 Based on Total Deal Value
Tags

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com