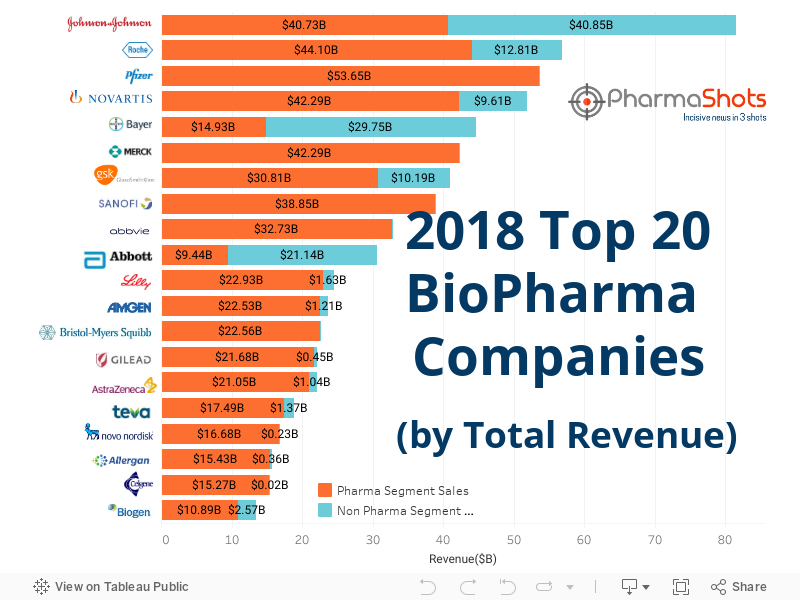
Top 20 BioPharma Companies based on 2018 Total Revenue
The global pharma companies have been advancing their pipelines with emerging needs of patients. Pharma companies are also focusing on diagnostics & devices- which were emerged as one of major revenue generator in 2018- with the development of new products and devices. The year 2018 also proved to be a record with approval of 55 novel drugs. The top 20 companies on the ledger changed from their 2017 ranking- leading to major ups & downs in the positions. Johnson and Johnson again proved to be the among the highest rank in 2018 generating revenue of $81.5B. Our team at PharmaShots has compiled a list of top 20 pharma companies based on their 2018 total revenue.
Total Revenue: $13.45B Total Employees: ~7-800
Pharma Segment Revenue: $10.86B Non-Pharma Segment Revenue: $2.56B
Founded Year: 1978 Headquarter: Massachusetts- United States
Market Cap: ~$46B Stock Exchange: NASDAQ
Biogen is a global biopharmaceutical company focusing on neurology with a broad portfolio including Tecfidera- Avonex- Plegridy- Tysabri and Fampyra. Biogen has expanded its business in 2018- with the acquisition of BIIB100 & BIIB110 candidates from Karyopharm and AliveGen respectively and further collaborated with Pfizer for BIIB100. The company has expanded its partnership with Ionis Pharmaceuticals with ten year agreement for the development of antisense oligonucleotide (ASO) drug candidates in neurological diseases. Biogen has also entered into a research collaboration with C4 Therapeutics (C4T) for its novel protein degradation platform.

Total Revenue: $15.42B Total Employees: ~16-962
Pharma Segment Revenue: $15.26B Non-Pharma Segment Revenue: $0.01B
Founded Year: 1986 Headquarter: New Jersey- United States
Market Cap: ~$66B Stock Exchange: NASDAQ
Celgene is an integrated global biopharmaceutical company focused on developing therapies for cancer & inflammatory diseases. Celgene's numerous key products are in P-III trials including Revlimid- Otezla- evaluated in patients with r/r follicular or marginal zone lymphoma & Behcet's disease respectively. In 2019- Celgene has been acquired by BMS for $74B.

Total Revenue: $15.78B Total Employees: ~17-000
Pharma Segment Revenue: $15.42B Non-Pharma Segment Revenue: $0.36B
Founded Year: 1950 Headquarter: Dublin- Ireland
Market Cap: ~$48B Stock Exchange: NYSE
Allergan is an Irish pharmaceutical company focused on developing & commercializing pharmaceuticals- devices- biologic- surgical and regenerative medicine for the people around the globe. Allergan didn't has much difference in revenue in comparison to its 2017 revenue. Allergan?s blockbuster products- Botox cosmetic- Botox therapeutic- Vraylar- and Juvederm added nearly $1B to its 2018 revenue.

Total Revenue: $16.90B Total Employees: ~43-202
Pharma Segment Revenue: $16.67B Non-Pharma Segment Revenue: $0.23B
Founded Year: 1989 Headquarter: Bagsværd, Denmark
Market Cap: ~$97B Stock Exchange: Copenhagen
Novo Nordisk is a global healthcare company focusing on therapies for Diabetes- Obesity- Hemophilia and growth disorders. 2018 was a progressive year for Novo Nordisk as it reached its target with the launch of Ozempic for the treatment of Type-2 Diabetes. Novo Nordisk is expected to increase its sales by 2-5% in 2019.

Total Revenue: $18.854B Total Employees: ~45-000
Pharma Segment Revenue: $17.48B Non-Pharma Segment Revenue: $1.36B
Founded Year: 1944 Headquarter: Petah Tikva- Israel
Market Cap: ~$16B Stock Exchange: NYSE
Teva Pharmaceutical is an Israeli multinational pharmaceutical company focused on generics- specialty medicines and biopharmaceuticals. Company's specialty portfolio includes Capaxone for multiple sclerosis- Ajovy for the treatment of migraine & Austedo for movement disorder. Teva's biosimilars (developed by Celltrion) were approved by the FDA in 2018- including Truxima & Herzuma biosimilar to rituximab & trastuzumab respectively.

Total Revenue: $22.09B Total Employees: ~50-000
Pharma Segment Revenue: $21.04B Non-Pharma Segment Revenue: $1.04B
Founded Year: 1999 Headquarter: Cambridge- United Kingdom
Market Cap: ~$105.8B Stock Exchange: LSE
AstraZeneca is a global- biopharmaceutical company focusing on Oncology- Respiratory- Neurology- Autoimmune and other therapy areas. In Dec'18 AstraZeneca and Merck's Lynparza (olaparib) received FDA's approval as 1L treatment for BRCA-Mutated Advanced Ovarian Cancer and signed an immune-oncology clinical collaboration with AVEO oncology. In May-18 AstraZeneca has also received FDA's approval for its Lokelma (sodium zirconium cyclosilicate) for the treatment of hyperkalemia in adults.

Total Revenue: $22.12B Total Employees: ~11-000
Pharma Segment Revenue: $21.67B Non-Pharma Segment Revenue: $0.45B
Founded Year: 1987 Headquarter: California- United States
Market Cap: ~$84B Stock Exchange: NASDAQ
Gilead Sciences is a research-based biopharmaceutical company with a broad portfolio of drugs. Biktarvy- Descovy- Odefsey- Genvoya- Stribild are approved in the US & EU for HIV infection in adults. Biktarvy received FDA's approval in Feb'18 for the treatment-naive HIV-1 infected in adults.

Total Revenue: $22.56B Total Employees: ~23-300
Pharma Segment Revenue: $22.56B Non-Pharma Segment Revenue: Nil
Founded Year: 1989 Headquarter: New York- United States
Market Cap: ~$75B Stock Exchange: NYSE
Bristol-Myers Squibb (BMS) is an American pharmaceutical company focused on Oncology- Cardiovascular- Immunology and Fibrosis. Its blockbuster drugs- Opdivo (nivolumab) for cancer & Eliquis as anticoagulant positioned BMS in the top 20 global pharma companies. BMS remained to be in the highlights- with its acquisition of Celgene in early 2019. The acquisition will boost up BMS' pipeline in Oncology- Immunology- Inflammation & Cardiology.

Total Revenue: $23.74B Total Employees: ~21-000
Pharma Segment Revenue: $22.53B Non-Pharma Segment Revenue: $1.2B
Founded Year: 1980 Headquarter: California- United States
Market Cap: ~$120B Stock Exchange: NASDAQ
Amgen is one of the leading biotechnology company developing novel therapies focused on Cardiology- Oncology- Neurology- Nephrology- and Inflammatory diseases. The company's total revenue increased by 7%- leading to a total increment of $6.2B. The company recently launched products Repatha (evolocumab)- Prolia (denosumab)- Kyprolis (carfilzomib) and Xgeva (denosumab) which presented a double- digit growth.

Total Revenue: $24.55B Total Employees: ~38-000
Pharma Segment Revenue: $22.93B Non-Pharma Segment Revenue: $1.62B
Founded Year: 1901 Headquarter: Indiana- United States
Market Cap: ~$139B Stock Exchange: NYSE
Eli Lilly and Company is a global pharmaceutical firm focused on developing therapies in the areas of diabetes- lung cancer- osteoporosis & men's health. In Sep'18- Lilly's animal health business Elanco completed its IPO on NYSE. In 2018- it had collaborated with Hydra Biosciences- AC immune to develop immune & neurodegenerativetherapies respectively. The FDA granted fast track designation to Lilly's Olumiant for SLE in Dec-18.

Total Revenue: $30.57B Total Employees: ~103-000
Pharma Segment Revenue: $9.43B Non-Pharma Segment Revenue: $21.14B
Founded Year: 1900 Headquarter: Illinois- United States
Market Cap: ~$130B Stock Exchange: NYSE
Abbott Laboratories is an American healthcare company with a broad pipeline of generics- diagnostic- natural products- Cardiovascular and Neuromodulation products. In 2018- Abbott received FDA's approval for the Advisor HD grid mapping catheter- sensor enabled MitraClip- a heart valve repair device- HeartMate 3 left ventricular assist device (LVAD)- XIENCE Sierra drug eluting stent system

Total Revenue: $32.73B Total Employees: ~30-000
Pharma Segment Revenue: $32.73B Non-Pharma Segment Revenue: Nil
Founded Year: 1900 Headquarter: Illinois- United States
Market Cap: ~$122B Stock Exchange: NYSE
AbbVie is a global- research-based biopharmaceutical company that develops therapies majorly for Chronic autoimmune diseases- Oncology- Virology with additional targets like cystic fibrosis and Women's health. In Oct-18 Health Canada approved AbbVie's Orilissa (elagolix) for pain associated with endometriosis. Its blockbuster product Humira (adalimumab) is a biologic therapy and is approved in multiple countries globally. In Dec,18- AbbVie pact a deal with Lupin to develop novel therapies for cancer.
Tags

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com