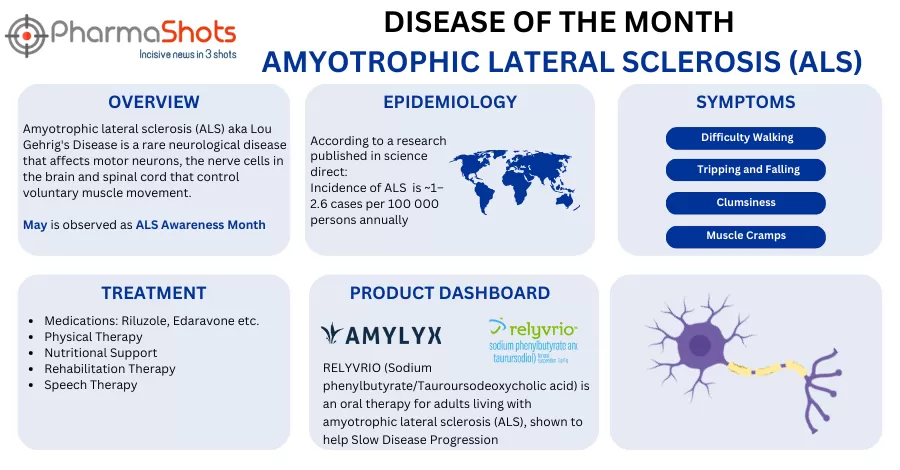
Amylyx Submits NDA to the US FDA for AMX0035 to Treat Amyotrophic Lateral Sclerosis
Shots:
- The NDA is based on CENTAUR trial evaluates AMX0035 vs PBO in 137 patients with ALS
- The results showed that patients receiving AMX0035 had a significant functional decline at the end of the 6mos. as measured by the ALSFRS-R. In an interim survival analysis @3yrs., 44% lower risk of death, median survival duration (25.0mos. & 18.5mos.), AEs and discontinuations were similar b/w AMX0035 and PBO groups 24wks.
- The company plans to submit an MAA for AMX0035 to the EMAs CHMP at the end of 2021 and plans to start a P-III clinical trial in the EU & US in Q421. The therapy is being evaluated in the P-III PHOENIX trial for ALS, based on the results from the CENTAUR trial
| Ref: Businesswire | Image: Amylyx
Click here to read the full press release
Tags

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com