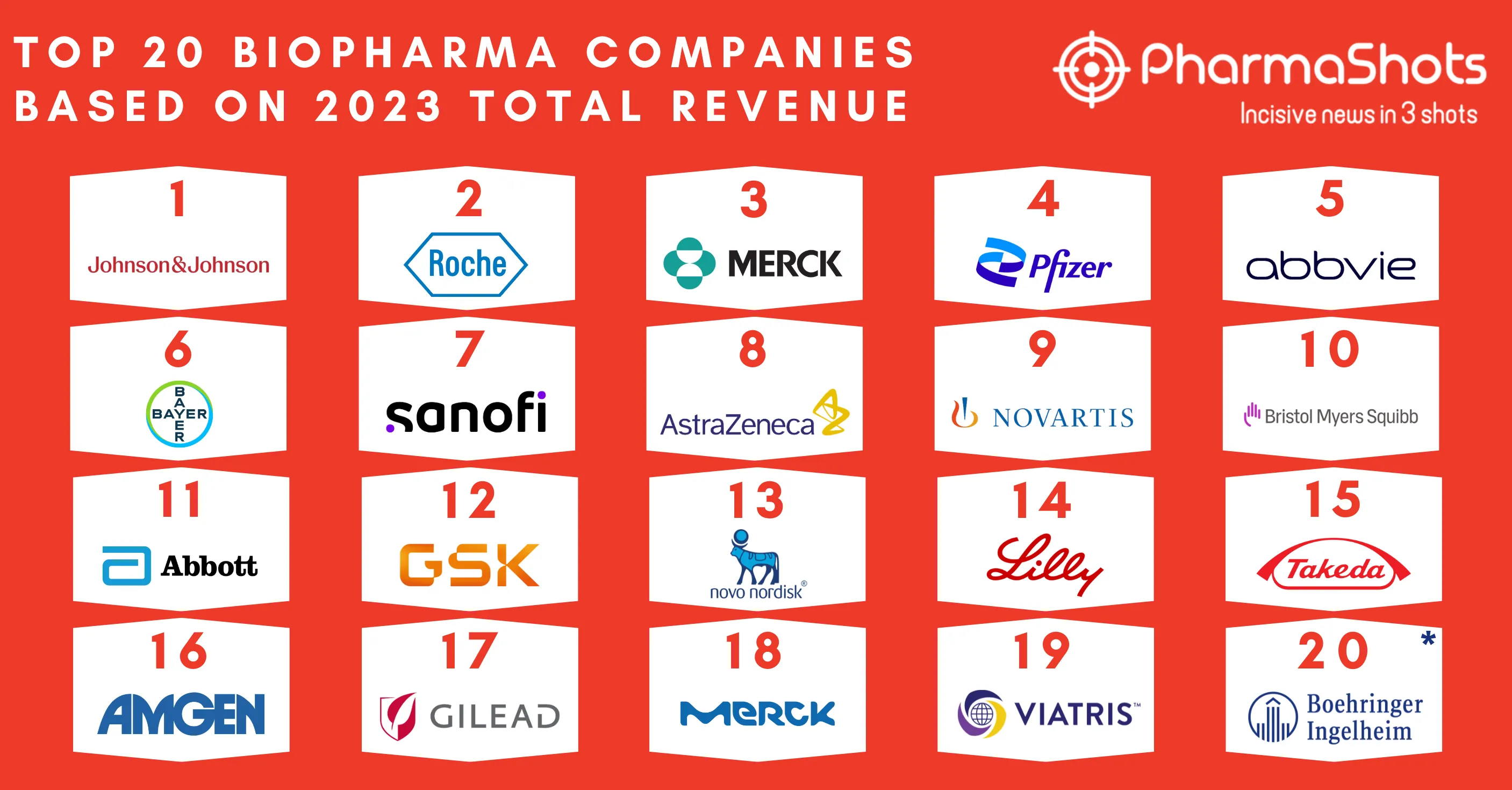
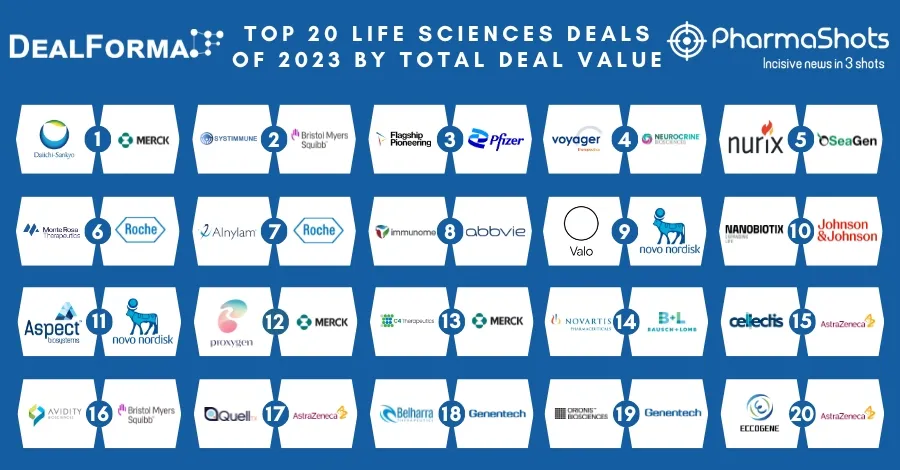
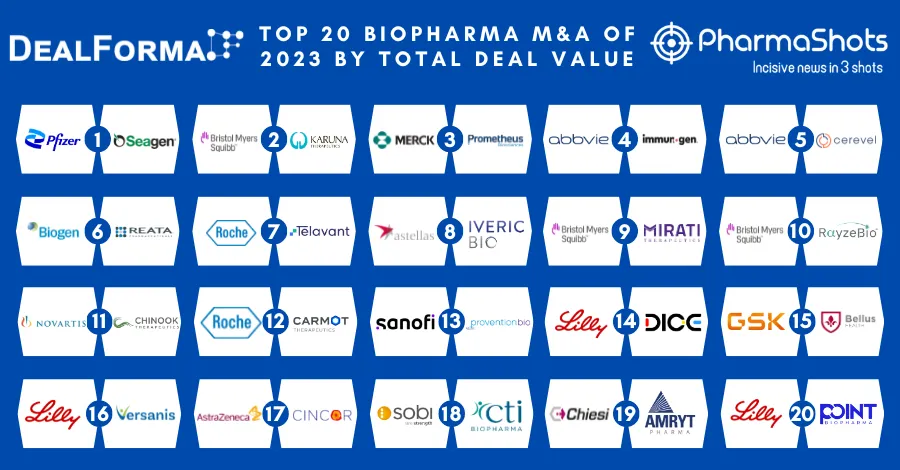
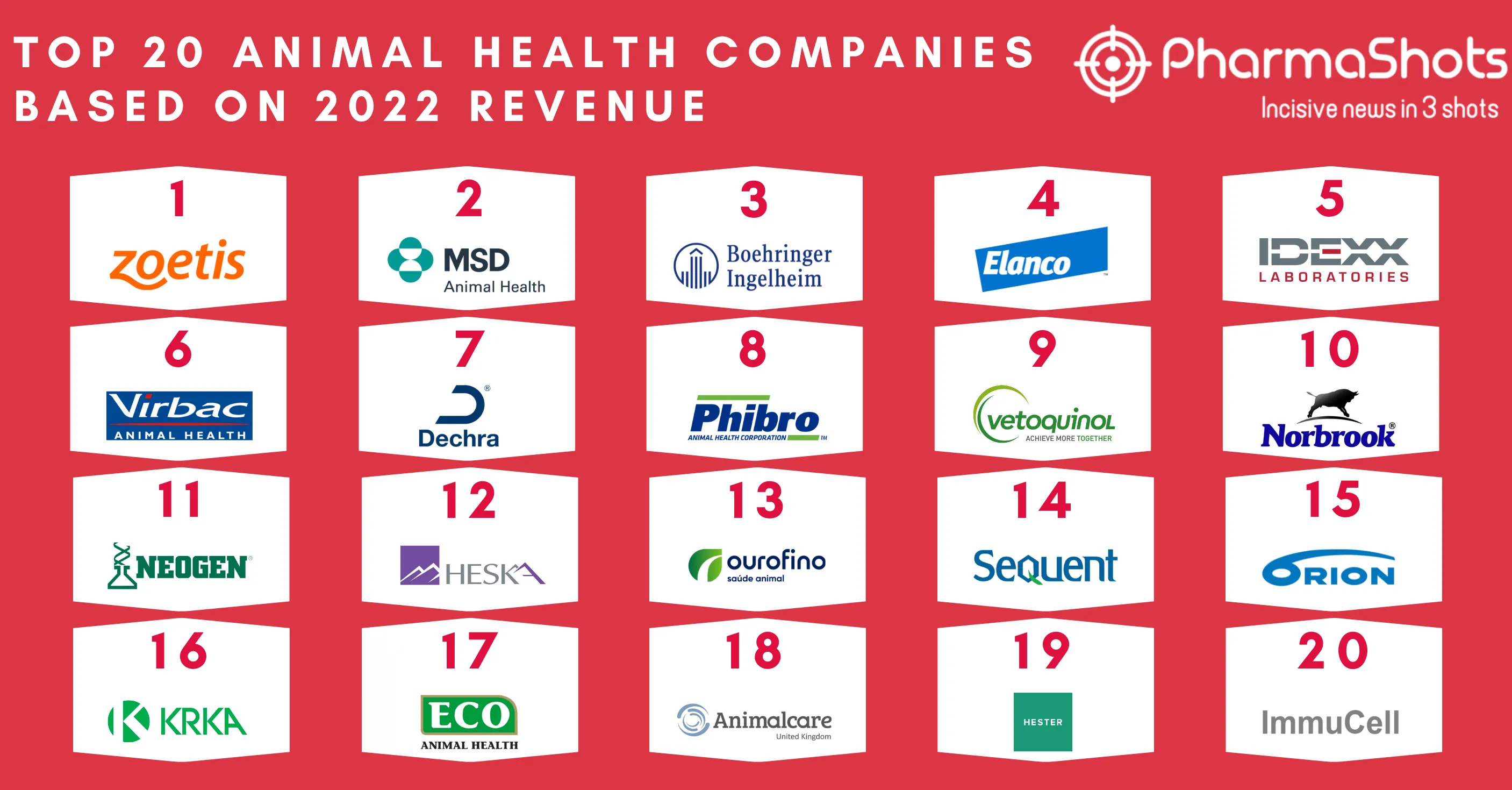
Eisai and Merck Annouces the US FDA Approval of Lenvima for Unresectable 1L Hepatocellular Carcinoma (HCC)
Shots:
- Approval is based on positive results of Ph III REFLECT study showing improved OS- PFS and ORR
- REFLECT results (Lenvima vs Sorafenib): mOS (13.6 vs 12.3 mos)- mPFS (7.3 vs 3.6 mos); ORR (41% vs 12%) with AE ≥20% patients & SAEs ≥2%
- Lenvima has been approved in Japan in H1’18 for HCC and was first approved in Feb’15 for thyroid cancer
Ref: Eisai | Image: Merck
Click here to read the full press release

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com