New Drug Designations – November 2025
Shots:
- PharmaShots’ Designation Report provides a concise overview of the latest drug designations granted by major regulatory authorities, including the FDA, EMA, MHLW, Health Canada, and NMPA
- The November 2025 report covers designations granted to 22 drugs and 1 medical device, spanning 8 small molecules, 8 biologics, 2 cell and gene therapies & 1 medical device, among others
- Significant trends this month show that BeOne Medicines’ sonrotoclax was granted priority review by the US FDA for the treatment of adults with r/r mantle cell lymphoma following treatment with a BTK inhibitor
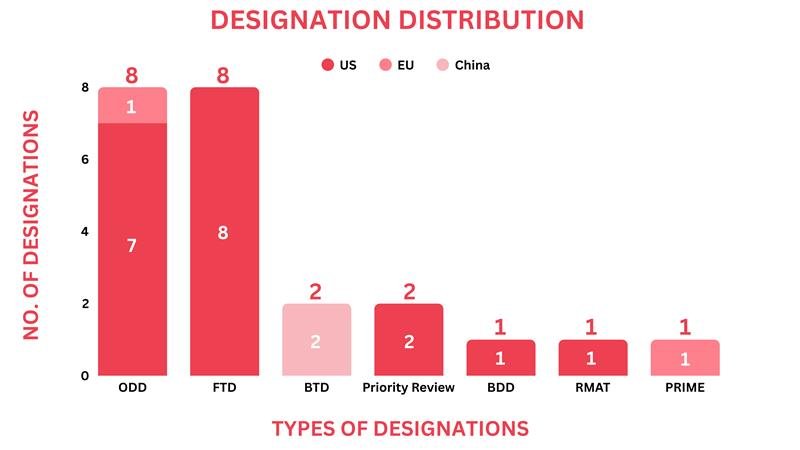

Drugs receiving orphan drug designation by global regulatory bodies echoed as small molecules, biologics, and recombinant proteins. Around 8 drugs received the orphan drug designation across multiple indications
A Quick Look
Small Molecule: Tinostamustine (Purdue Pharma) for Malignant Gliomas, NBM-BMX (NovelWise Pharmaceutical) for Uveal Melanoma, MIV-711 (Medivir) for Osteogenesis Imperfecta, Desidustat (Zydus) for Beta Thalassemia, Biologic: ABBV-CLS-628 (Calico Life Sciences) for Autosomal Dominant Polycystic Kidney Disease, OBI-902 (OBI Pharma) for Cholangiocarcinoma, Plasma-derived Therapy: Ceruloplasmin Therapy (Kedrion) for Aceruloplasminemia, Recombinant Proteins: M2T-CD33 (Leukogene Therapeutics) for Acute Myeloid Leukemia

Around 8 drugs received the Fast Track Designation as small molecules, biologics, and peptides
A Quick Look
Small Molecule: Zotiraciclib (Cothera Bio) for Recurrent high-grade Gliomas, VS-041 (Vasa Therapeutics) for Heart Failure with Preserved Ejection Fraction, DPTX3186 (Dewpoint Therapeutics) for Gastric Cancer, Biologic: 4A10 (Allterum Therapeutics) for R/R Acute Lymphoblastic Leukemia, AVZO-1418 (Avenzo Therapeutics) for EGFR-Mutated TKI-Pretreated NSCLC, AVZO-103 (Avenzo Therapeutics) for Urothelial Cancer, Radiopharmaceutical: ITM-94 (ITM Isotope Technologies) for Clear Cell Renal Cell Carcinoma, Peptide – FOG-001 (Parabilis Medicines) for Desmoid Tumors

Regulatory bodies designated two drugs with Breakthrough Therapy Designation, both biologics
A Quick Look
Biologic: Ivonescimab (Akeso) for Triple-Negative Breast Cancer, Serplulimab (Henlius) for Gastric Cancer

Two drugs were given priority review, including a small molecule and a biologic
A Quick Look
Small Molecule: Sonrotoclax (BeOne Medicine) for R/R Mantle Cell Lymphoma, Biologic: Dupixent (Regeneron & Sanofi) for Allergic Fungal Rhinosinusitis

One device received the breakthrough device designation by the US FDA
A Quick Look
Molecular Diagnostic Platform (Nanopath) for Urinary Tract Infection Diagnosis

One drug was given the PRIME designation as a Cell Therapy
A Quick Look
Cell Therapy: NRTX-1001 (Neurona Therapeutics) for Drug-resistant focal epilepsy

One drug received the regenerative medicine advanced therapy designation
A Quick Look
Cell Therapy: MB-105 (March Biosciences) for R/R CD5-positive T-cell Lymphoma
Related Post: New Drug Designations – October 2025



