The US FDA New Drug Approvals in November 2025
Shots:
- Innovation surged in November, with cutting-edge science and strategic collaborations reshaping the drug development landscape across oncology, renal disease, hereditary conditions, and more
- The US FDA cleared five standout therapies, including Bayer’s Hyrnuo (Sevabertinib), UCB’s Kygevvi (Doxecitine + Doxribtimine), and Arrowhead’s Redemplo, underscoring a month of meaningful clinical progress
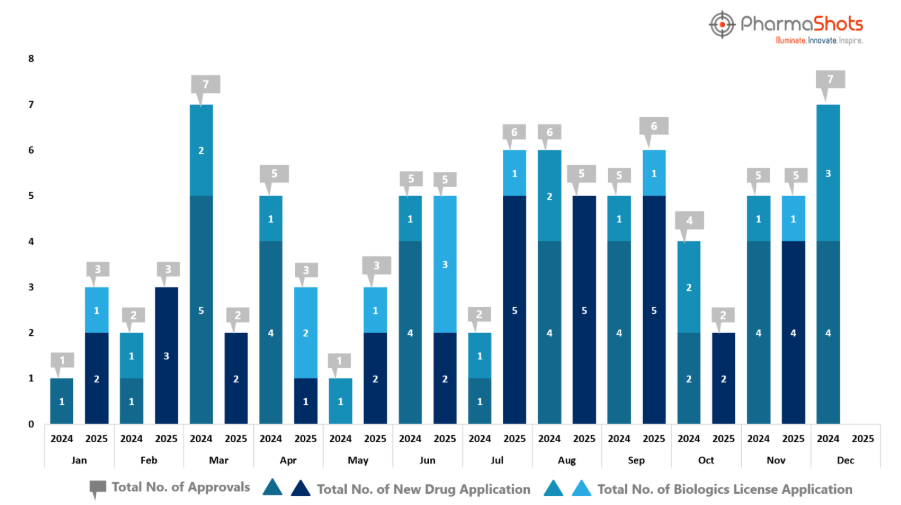
- November 2025 closed with five approvals, matching last year’s total, signaling sustained momentum and a steady flow of breakthrough treatments entering the market
Company: UCB
Product: Kygevvi
Active Ingredient: Doxecitine + Doxribtimine
Disease: Thymidine Kinase 2 Deficiency
Date: Nov 03, 2025
Shots:
- FDA has approved Kygevvi (2g/2g; PO) for adults & pediatric pts with TK2d whose symptom onset occurred ≤12yrs. of age. Kygevvi is expected to launch in the US by Q1’26, & is under EMA’s review, with additional regulatory filings planned
- Approval was backed by a P-II trial, 2 retrospective chart review studies, & an expanded access use program comparing Kygevvi vs pyrimidine nucleosides in 82 pts, with OS assessed against untreated controls who were matched to treated pts using age of TK2d symptom onset
- Among 78 matched pairs, Kygevvi reduced the risk of death by ~86%, with a median TK2d onset at 1.5yrs., a median treatment duration of 4yrs., & a median dose of 762 mg/kg/day
Company: Kura Oncology and Kyowa Kirin
Product: Komzifti
Active Ingredient: Ziftomenib
Disease: NPM1-Mutated Acute Myeloid Leukemia
Date: Nov 13, 2025
Shots:
- The US FDA has granted full approval to Komzifti (QD, PO) to treat adults with r/r AML with NPM1 mutation who have no satisfactory alternative treatment options before the PDUFA date of Nov 30, 2025
- Approval was backed by P-I/II (KOMET-001) trial in 112 r/r NPM1-mutant AML pts, showing 21.4% CR + CRh with a median duration of 5mos., & a median time to first response of 2.7mos. in those who achieved CR or CRh, with 88% responding within 6mos.; data published in The JCO
- In Nov 2024, Kura & Kyowa formed a global collaboration for Komzifti, with Kura leading US development, regulatory, commercialization, & manufacturing, jointly handling some US commercialization activities, while Kyowa Kirin leads all strategy & commercialization outside the US
Company: Arrowhead Pharmaceuticals
Product: Redemplo
Active Ingredient: Plozasiran
Disease: Familial Chylomicronemia Syndrome
Date: Nov 18, 2025
Shots:
- The US FDA has approved Redemplo as an adjunct to diet to reduce triglycerides (TGs) in adults with FCS; US availability is expected by year-end
- Approval was based on the P-III (PALISADE) trial assessing Redemplo (25mg & 50mg, SC, Q3M) vs PBO in 75 adults with genetically confirmed/clinically diagnosed FCS. Pts could join a 2-part extension period where all receive Redemplo after completing randomization
- Trial met its 1 & key 2EPs, with reductions in TGs & APOC3. Redemplo (25mg) showed an -80% median TG reduction vs -17% in the pooled PBO group & demonstrated a lower numerical rate of acute pancreatitis; data was published in The NEJM & Circulation, & presented at ESC’24 & AHA’24
Company: Bayer
Product: Hyrnuo
Active Ingredient: Sevabertinib
Disease: Advanced HER2-mutant NSCLC
Date: Nov 19, 2025
Shots:
- The US FDA has granted accelerated approval to Hyrnuo (BAY 2927088; reversible TKI) under priority review for the treatment of previously treated pts with LA/M NSCLC harboring HER2 tyrosine kinase domain activating mutations; NDA under NMPA’s review
- Approval was based on the ORR & DoR data from the ongoing P-I/II (SOHO-01) trial assessing Hyrnuo (PO) in HER2-mutant NSCLC pts who had disease progression after ≥1 systemic therapy
- Trial showed ORR of 71% (n=70), with CR of 2.9% & PR of 69% as well as mDoR of 9.2mos. (n=50) in pts naïve to HER2 targeted therapy, plus); data was presented at ESMO’25 & published in The NEJM
Company: Otsuka Pharmaceutical
Product: Voyxact
Active Ingredient: Sibeprenlimab-szsi
Disease: Primary Immunoglobulin A Nephropathy
Date: Nov 25, 2025
Shots:
- The US FDA has granted accelerated approval to Voyxact (sibeprenlimab-szsi) for the reduction of proteinuria in adults with primary IgAN at risk for disease progression
- Approval was based on the interim data from the ongoing P-III (VISIONARY) trial, assessing Voyxact (400mg, SC, Q4W) vs PBO in 510 IgAN adults, who were on SoC therapy
- Trial met its 1EP, showing a PBO-adjusted 51% reduction in proteinuria at 9mos. (n=320), with eGFR decline data expected in early 2026 & it will support traditional FDA approval
Related Post: Insights+: The US FDA New Drug Approvals in October 2025



