New Drug Designations – October 2025
Shots:
- PharmaShots’ Designation Report provides a concise overview of the latest drug designations granted by major regulatory authorities, including the FDA, EMA, MHLW, Health Canada, and NMPA
- The October 2025 report covers designations granted to 56 drugs and 9 medical devices, spanning 23 small molecules, 14 biologics, 10 cell and gene therapies & 9 medical devices, among others
- Significant trends this month show, Blacksmith Medicines’ FG-2101 secured Fast Track and Qualified Infectious Disease Product Designations under the GAIN Act for the treatment of serious infections caused by Gram-negative bacteria, incl. multidrug-resistant strains
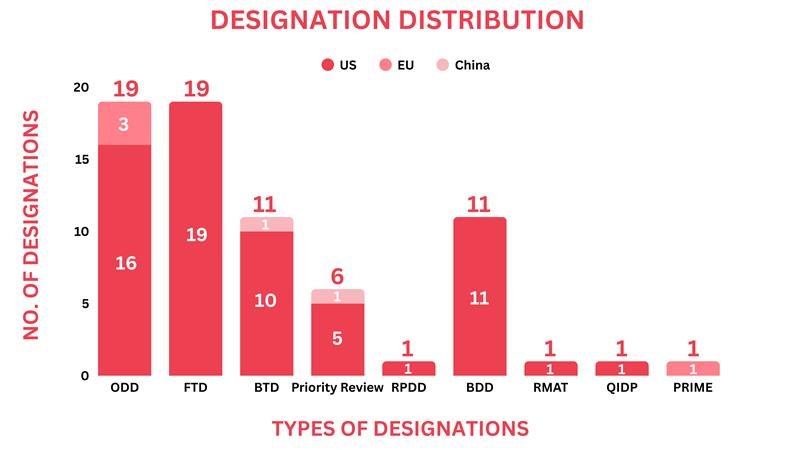

Drugs receiving orphan drug designation by global regulatory bodies echoed as small molecules, biologics, and antisense oligonucleotides. Around 19 drugs received the orphan drug designation across multiple indications
A Quick Look
Small Molecule: QRX003 (Quoin Pharmaceuticals) for Netherton Syndrome, PORT-77 (GondolaBio & Portal Therapeutics) for Erythropoietic Protoporphyria & X-linked Protoporphyria, Ofirnoflast (Halia Therapeutics) for Myelodysplastic Syndromes, AMXT 1501 (Aminex Therapeutics) for Neuroblastoma, Daraxonrasib (Revolution Medicines) for Pancreatic Cancer, DPTX3186 (Dewpoint Therapeutics) for Gastric Cancer, ZEN-3694 (Zenith Epigenetics) for NUT carcinoma, Biologic: KPL-387 (Kiniksa Pharmaceuticals) for Pericarditis, HLX43 (Henlius) for Thymic Epithelial Tumors, GSK5764227 (GSK) for Pulmonary neuroendocrine Carcinoma, CAL101 (Calluna Pharma) for Idiopathic pulmonary fibrosis, Antivirals: RBD1016 (Suzhou Ribo Life Science) for Hepatitis D Virus, Gene Therapy: NEU-001 (Neurenati Therapeutics) for Hirschsprung disease, AAVB-039 (AAVantgarde) for Stargardt disease, Nanoparticle: CNP-106 (COUR Pharma) for Generalized Myasthenia Gravis, Cell Therapy: CK0803 (Cellenkos) for Amyotrophic Lateral Sclerosis, MNV-201 (Minovia Therapeutics) for Myelodysplastic Syndrome, Fusion Protein: IL13PE38 (Precision NeuroMed) for Glioblastoma Multiforme, Antisense Oligonucleotides: HT-KIT (Hoth Therapeutics) for c-KIT-Driven Cancers

Around 19 candidates, including a drug-device combination, received the Fast Track Designation as gene therapy, small molecules, biologics, and cell therapy
A Quick Look
Small Molecule: PORT-77 (GondolaBio & Portal Therapeutics) for Erythropoietic Protoporphyria & X-linked Protoporphyria, ALTO-101 (Alto Neuroscience) for cognitive impairment associated with Schizophrenia, ETX-636 (Ensem Therapeutics) for PIK3CA-mutant Breast Cancer, MT-125 (Myosin Therapeutics) for Glioblastoma, VT3989 (Vivace Therapeutics) for Nonpleural or Pleural Mesothelioma, FG-2101 (Blacksmith Medicines) for Gram-negative bacteria infections, NBM-BMX (Novelwise Pharmaceutical) for Metastatic Uveal Melanoma, Biologic: ADCE-D01 (Adcendo) for soft tissue sarcoma, HDP-101 (Heidelberg Pharma) for r/r multiple myeloma, BMS-986446 (BMS) for Early Alzheimer’s Disease, JSKN003 (Alphamab) for platinum-resistant recurrent epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer, ABBV-CLS-628 (Calico Life Sciences) for Autosomal Dominant Polycystic Kidney Disease, Scp776 (Silver Creek Pharmaceuticals) for Acute Ischemic Stroke, Peptide: EO2463 (Enterome) for follicular lymphoma, Immunotherapy: WTX-124 (Werewolf Therapeutics) for Cutaneous Melanoma, Gene Therapy: EG110A (EG 427) for Neurogenic detrusor overactivity, NG-350A (Akamis Bio) for Mismatch Repair-Proficient Locally Advanced Rectal Cancer, Cell Therapy: ISP-001 (Immusoft) for Mucopolysaccharidosis type I, Drug-Device Combination: EpiSmart Epithelium-On Cross-Linking System for Keratoconus

Regulatory bodies designated eleven drugs with Breakthrough Therapy Designation, ranging from Peptide and Antisense oligonucleotides to biologics
A Quick Look
Small Molecule: Sonrotoclax (BeOne Medicine) for R/R Mantle Cell Lymphoma, Bezuclastinib (Cogent Biosciences) for NonAdvanced Systemic Mastocytosis & Smoldering Systemic Mastocytosis, NPI-001 (Nacuity Pharmaceuticals) for Retinitis pigmentosa, CD 388 (Cidara Therapeutics) for Influenza, BPL-003 (atai Life Sciences & Beckley Psytech) for Depression, Recombinant Fusion Proteins: Ficerafusp Alfa (Bicara Therapeutics) for Head and Neck Squamous Cell Carcinoma, Tralesinidase Alfa (Spruce Biosciences) for Sanfilippo Syndrome Type B, Gene Therapy: TSHA-102 (Taysha Gene Therapies) for Rett Syndrome, Biologic: Zenocutuzumab-zbco (Partner Therapeutics) for NRG1+ Cholangiocarcinoma, ELA026 (Electra Therapeutics) for Secondary Hemophagocytic Lymphohistiocytosis, Gotistobart (OncoC4 & BioNTech) for squamous NSCLC

Six drugs were given priority review, ranging from small molecules, cell therapy to biologics
A Quick Look
Small Molecule: Leniolisib (Pharming Group) for Activated phosphoinositide 3-kinase delta syndrome, Akeega (J&J) for BRCA-mutated metastatic castration-sensitive prostate cancer, Tinlarebant (Belite Bio) for Stargardt disease, Cell Therapy: Orca-T (Orca Bio) for Hematological Malignancies, Biologic: Padcev + Keytruda (Pfizer, Astellas & Merck) for Muscle-Invasive Bladder Cancer, Enzyme: Palynziq (BioMarin Pharmaceutical) for Phenylketonuria

One drug received the rare pediatric disease designation as a radiopharmaceutical small molecule
A Quick Look
Small Molecule: Iopofosine I 131 (Cellectar) for R/R Pediatric High-Grade Glioma

Over nine devices received the breakthrough device designation by the US FDA
A Quick Look
Syn-One Test (CND Life Sciences) for Neurologic conditions, Obstructive Hydrocephalus Triage and Prioritization Solution (Harrison.Ai) for CT Imaging, Rapid Bloodstream Infection Assay (Scanogen) for Pathogen Detection, PlasmaSure (CAPS Medical) for Non-Muscle Invasive Bladder Cancer, Injectable Electrode Technology (Rhythio Medical) for Defibrillation, ExomeDx and GenomeDx Testing (GeneDx) for Genetic disorders, Avvio Enhanced Lithotripsy System (Avvio Medical) for Kidney Stone Treatment, iTaperloc Complete and iG7 Hip System (Zimmer Biomet) for Hip Replacement, DPYD Genotyping Assay (APIS Assay Technologies) for DPYD genotyping

One drug received the Qualified Infectious Diseases Product Designation
A Quick Look
Small Molecule: FG-2101 (Blacksmith Medicines) for Gram-negative bacteria infections

One drug received the PRIME designation by the EMA
A Quick Look
Biologic: ELA026 (Electra Therapeutics) for Secondary Hemophagocytic Lymphohistiocytosis

One drug received the regenerative medicine advanced therapy designation
A Quick Look
Cell Therapy: AVB-114 (Avobis Bio) for Crohn’s Perianal Fistulas
Related Post: New Drug Designations – September 2025




