The US FDA New Drug Approvals in October 2025
Shots:
- Innovation in drug development continues to shape the future of healthcare, driving bold scientific collaborations and transforming the way diseases are treated across every frontier of medicine
- In October, the US FDA granted approvals to Boehringer Ingelheim’s Jascayd (nerandomilast) for adults with idiopathic pulmonary fibrosis and Bayer’s Lynkuet (elinzanetant) for managing moderate to severe vasomotor symptoms associated with menopause
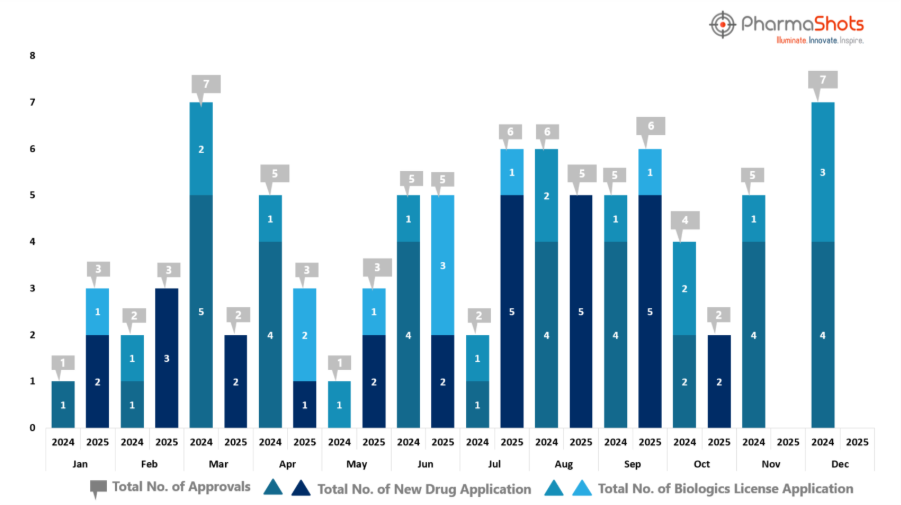
- While October 2025 saw two new drug approvals, compared to four in October 2024, the decline reflects a strategic recalibration in R&D and regulatory focus, signaling a shift toward more specialized therapies and precision-driven development pathways that are redefining innovation priorities across the industry
Company: Boehringer Ingelheim
Product: Jascayd
Active Ingredient: Nerandomilast
Disease: Idiopathic Pulmonary Fibrosis
Date: Oct 07, 2025
Shots:
- The US FDA has approved Jascayd (nerandomilast) for the treatment of adults with idiopathic pulmonary fibrosis (IPF); regulatory review is ongoing in China, Japan, & the EU, with further filings planned
- Approval was based on P-III (FIBRONEER-IPF) & P-II (Trial 2) trials, where FIBRONEER-IPF assessed Jascayd (18 or 9mg, PO, BID) vs PBO in IPF pts for atleast or over 52wks.
- Trial met its 1EP, showing reduced FVC decline at 52wks. (-106 mL & -122 mL vs -170 mL), with the 18 mg dose demonstrating benefit as early as Wk. 2 & sustained divergence through Wk. 52
Company: Bayer
Product: Lynkuet
Active Ingredient: Elinzanetant
Disease: Vasomotor Symptoms
Date: Oct 24, 2025
Shots:
- The US FDA has approved Lynkuet for the treatment of mod. to sev. vasomotor symptoms due to menopause based on P-III (OASIS-1, 2, & 3) trials, with commercial availability expected at the start of Nov 2025; MAA is under the EMA’s review
- Efficacy was evaluated in OASIS-1 & 2 trials assessing Lynkuet vs PBO in 796 menopausal women, meeting their 1EP of reduced mod. to sev. VMS at Wks. 4 & 12, incl. day and night hot flashes
- Safety was assessed in P-III (OASIS-1, 2 & 3) trials involving 1,420 women, incl. 627 in OASIS 3 who received Lynkuet or PBO for up to 52wks. to assess long-term safety
Related Post: Insights+: The US FDA New Drug Approvals in September 2025




