New Drug Designations – September 2025
Shots:
- PharmaShots’ Designation Report provides a concise overview of the latest drug designations granted by major regulatory authorities, including the FDA, EMA, MHLW, Health Canada, and NMPA
- The September 2025 report covers designations granted to 33 drugs and 4 medical devices, spanning 12 small molecules, 6 biologics, 8 cell and gene therapies & 4 medical devices among others
- Significant trends this month show, Nanoscope Therapeutics’s MCO-010 (sonpiretigene isteparvovec) secured 5 Orphan Drug Designations from EMA for retinal diseases and a Regenerative Medicine Advanced Therapy Designation from the US FDA for Stargardt disease
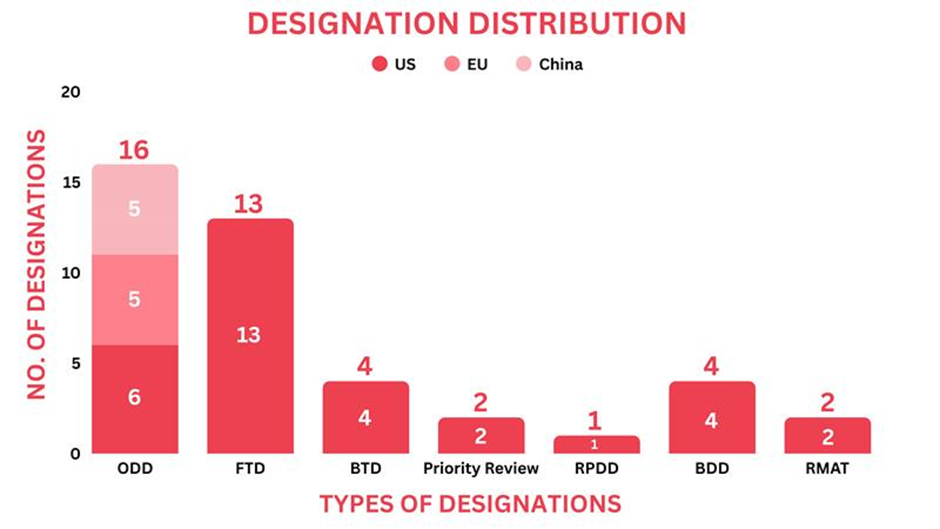

Drugs receiving orphan drug designation by global regulatory bodies echoed as small molecules, biologics, and antisense oligonucleotides. Around 12 drugs received the orphan drug designation across multiple indications.
A Quick Look
Small Molecules: RGT-61159 (Rgenta Therapeutics) for Adenoid Cystic Carcinoma, BA-101 (Neuro NOS) for Glioblastoma, DMX-200 (Dimerix) for Focal Segmental Glomerulosclerosis, Mosliciguat (Pulmovant) for Pulmonary Hypertension Associated with Interstitial Lung Disease, Buloxibutid (Vicore Pharma) for Idiopathic Pulmonary Fibrosis, Utidelone (Biostar Pharma) for Pancreatic Cancer, Biologic: Ligufalimab (Akeso) for Acute Myeloid Leukemia, Gene Therapy: CRD-003 (Cure Rare Disease) for Limb-Girdle Muscular Dystrophy Type R9, MCO-010 (Nanoscope Therapeutics) across 5 categories of Retinal Dystrophies, Enzyme: JR-446 (JCR Pharmaceuticals) for Mucopolysaccharidosis Type IIIB, Antisense Oligonucleotide: NS-051 (NS Pharma) for Duchenne Muscular Dystrophy, DYNE-251 (Dyne Therapeutics) for Duchenne Muscular Dystrophy

Around 13 drugs received the Fast Track Designation as gene therapy, small molecules, biologics, & cancer vaccine.
A Quick Look
Cancer Vaccine: GLSI-100 (Greenwich LifeSciences) for HLA-A*02 genotype and HER2-positive Breast Cancer, Small Molecule: NS-229 (NS Pharma) for Eosinophilic Granulomatosis with Polyangiitis, Emrusolmin (Teva) for Multiple System Atrophy, Alnodesertib (Artios Pharma) for ATM-negative Metastatic Colorectal Cancer, CS-1103 (Clear Scientific) for Methamphetamine Intoxication, Gene Therapy: SAR402663 (Sanofi) for Neovascular AMD, MVX-220 (MavriX Bio) for Angelman Syndrome, SAR446268 (Sanofi) for Myotonic Dystrophy Type 1, CER-1236 (CERo Therapeutics) for Acute Myeloid Leukemia, Immunotherapy: MNV-201 (Minovia Therapeutics) for Myelodysplastic Syndrome, Biologic: CRB-701 (Corbus Pharmaceuticals) for Head and Neck Squamous Cell Carcinoma, Etalanetug (Eisai) for Alzheimer’s Disease, Cell Therapy: UB-VV111 (Umoja Biopharma) for R/R B-cell Malignancies & CLL

Regulatory bodies designated seven drugs with Breakthrough Therapy Designation, ranging from Peptide and Antisense oligonucleotides to biologics.
A Quick Look
Small Molecule: Hernexeos (Boehringer Ingelheim) for HER2-mutant NSCLC, Olomorasib (Eli Lilly) for KRAS G12C-mutant NSCLC, Antisense Oligonucleotide: ION582 (Ionis) for Angelman Syndrome, Biologic: Raludotatug Deruxtecan (Merck) for Ovarian & Related Cancers

Two drugs, one a small molecule and the other a Biologic, were given priority review.
A Quick Look
Small Molecule: Idebenone (Chiesi Global Rare Diseases) for Leber Hereditary Optic Neuropathy, Biologic: Enhertu + Perjeta (Daiichi Sankyo & AstraZeneca) for HER2+ Breast Cancer

One drug received the rare pediatric disease designation as an antisense oligonucleotide
A Quick Look
Biologic: ART5803 (Arialys Therapeutics) for Anti-NMDA Receptor Encephalitis

Four devices received the breakthrough device designation by the US FDA
A Quick Look
Sentante Stroke System, Virtuoso Surgical Robotic System, Multi-triage Solution, Non-Contact Vascular Access (VA) Management Technology

Two drugs received the regenerative medicine advanced therapy designation
A Quick Look
Gene Therapy: MCO-010 (Nanoscope Therapeutics) for Stargardt disease, ETX101 (Encoded Therapeutics) for SCN1A+ Dravet Syndrome
Related Post: New Drug Designations – August 2025




