EMA Marketing Authorization of New Drugs in September 2025
Shots:
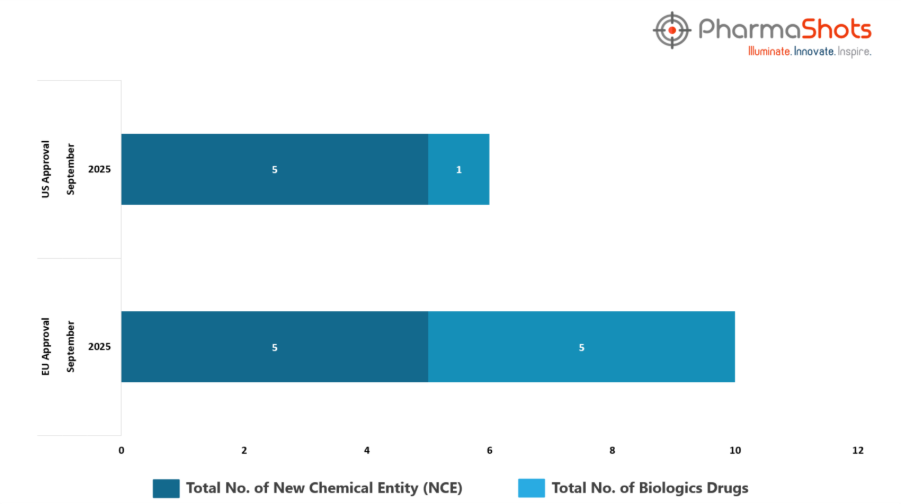
- The EMA’s CHMP has granted approvals to 5 Biologics and 5 new chemical entities in September 2025, leading to treatments for patients and advances in the healthcare industry
- The major highlighted drug, Merck’s Enflonsia, has Adopted Positive Opinion for RSV Prevention in Infants
- PharmaShots has compiled a list of 9 drugs that have been approved and recommended by the EC and CHMP, respectively
Company: Deciphera Pharmaceuticals
Product: Romvimza
Active Ingredient: Vimseltinib
Disease: Symptomatic Tenosynovial Giant Cell Tumor
Date: Sep 18, 2025
Shots:
- The EC has approved Romvimza (vimseltinib) to treat symptomatic TGCT with physical function deterioration in adults for which surgery may lead to functional impairment or severe morbidity
- Approval was based on P-I/II trial as well as P-III (MOTION) trial, which evaluated Romvimza vs PBO in surgery-ineligible pts without prior anti-CSF1/CSF1R therapy (prior imatinib/nilotinib allowed)
- The P-III trial met its 1EP, plus showed improvements across all 2EPs incl. ORR per TVS, active ROM, physical function, stiffness, QoL & pain at 25wks., while in a descriptive analysis at 97wks., 19/83 pts achieved CR per RECIST v1.1, with median time to CR of 11.5mos.
Company: Biogen
Product: Zurzuvae
Active Ingredient: Zuranolone
Disease: Postpartum Depression
Date: Sep 17, 2025
Shots:
- The EC has approved Zurzuvae (zuranolone) for the treatment of postpartum depression in adults following MHRA’s approval in Aug 2025
- Approval was based on P-III (SKYLARK) trial assessing Zurzuvae (50mg) vs PBO in pts with severe postpartum depression
- Trial met its 1EP with a significant mean reduction in HAMD-17 total score at Day 15, & all key 2EP, which showed a rapid reduction in depressive symptoms by Day 3, sustained through Day 45
Company: Merck
Product: Enflonsia
Active Ingredient: Clesrovimab
Disease: RSV Lower Respiratory Tract Disease
Date: Sep 18, 2025
Shots:
- The CHMP has recommended Enflonsia to prevent RSV lower respiratory tract disease in newborns & infants entering their first RSV season, with potential approval valid in all 30 EEA states
- Opinion was based on P-IIb/III (CLEVER) study assessing Enflonsia in preterm & full-term infants (≤1yr.), plus P-III (SMART) study of Enflonsia vs palivizumab in high-risk infants; data was published in The NEJM
- Enflonsia (105mg/0.7 mL, PFS) is a long-acting preventive monoclonal antibody designed to deliver immediate & sustained protection for up to 5mos., with a single weight-independent dose
Company: Bayer
Product: Lynkuet
Active Ingredient: Elinzanetant
Disease: Vasomotor Symptoms
Date: Sep 18, 2025
Shots:
- The CHMP has recommended elinzanetant for treating mod. to sev. vasomotor symptoms linked with menopause or caused by AET related to breast cancer based on P-III (OASIS-1, 2, 3 & 4) trials
- OASIS-1 & 2 showed reduced mod. to sev. menopausal VMS vs. PBO at wks. 4 & 12, with >80% pts (incl. those who switched from PBO) achieved ≥50% VMS reduction by wk. 26, while meeting all 3 key 2EPs of reduced VMS frequency, improved sleep & QoL; published in The JAMA
- OASIS-3 also depicted reduced mod. to sev. VMS vs. PBO over 12wks. with benefits maintained over 52wks. (published in The JAMA Internal Medicine), while OASIS-4 showed similar VMS, sleep, & QoL improvements in women on HR+ breast cancer endocrine therapy (published in The NEJM)
Company: Johnson & Johnson
Product: Imaavy
Active Ingredient: Nipocalimab
Disease: Generalized Myasthenia Gravis
Date: Sep 18, 2025
Shots:
- The CHMP has recommended Nipocalimab as an add-on therapy to treat anti-AChR Ab+ & anti-MuSK Ab+ gMG pts (≥12yrs.)
- Opinion was based on the P-III (Vivacity-MG3) trial assessing nipocalimab (30mg/kg, IV loading dose then 15mg/kg, Q2W) + SoC vs PBO + SoC in gMG pts (N=199; 153 were Ab +ve), which showed improved MG-ADL score over 24wks. (1EP), sustained for ~84wks. in the OLE phase
- Recommendation also incl. data from P-II/III (Vibrance-MG) pediatric trial, which met its 1EP of reduced IgG levels with nipocalimab (IV, Q2W), & achieved its 2EPs of improved MG-ADL & QMG scores
Company: KalVista Pharmaceuticals
Product: Ekterly
Active Ingredient: Sebetralstat
Disease: Hereditary Angioedema
Date: Sep 19, 2025
Shots:
- The EC and Swissmedic have approved Ekterly to treat acute HAE attacks in pts (age≥12) across EEA states. Launch is expected in Germany in Q4’25 and in Switzerland in H2’26
- Ekterly’s approval by the EC and Swissmedic was based on the P-III (KONFIDENT) trial. Published in NEJM (May 2024), results showed significantly faster symptom relief, reduced attack severity, and resolution vs. PBO, with a well-tolerated safety profile. The trial included 136 pts across 20 countries
- Ekterly, plasma kallikrein inhibitor, is approved in the US, EU, UK, and Switzerland for treating HAE attacks
Company: Servier
Product: Voranigo
Active Ingredient: Vorasidenib
Disease: Grade 2 IDH-mutant glioma
Date: Sep 19, 2025
Shots:
- The EC has approved Voranigo for treating patients (age: ≥12yrs.; wt≥ 40kg) with Gr2 astrocytoma or oligodendroglioma with a susceptible IDH1/2 mutation post-surgery, but do not currently require radiotherapy or CT across EEA states
- Approval was based on the global pivotal P-III (INDIGO) trial, which demonstrated that vorasidenib significantly improved PFS and TTNI vs PBO The median PFS (27.7mos. vs 11.1mos.), and TTNI was also significantly prolonged, with the mTTNI not reached vs 17.8mos. An exploratory EP showed reduced tumor volume by an average of 2.5% vs 13.9% every 6mos.
- Safety profile consistent with P-I studies. Published in The NEJM and presented at the ASCO’23
Company: Ionis Pharmaceuticals
Product: Tryngolza
Active Ingredient: Olezarsen
Disease: Familial Chylomicronemia Syndrome
Date: Sep 19, 2025
Shots:
- The EC has approved Tryngolza (olezarsen) as an adjunct to diet in adult patients for the treatment of genetically confirmed familial chylomicronemia syndrome
- Approval was based on P-III (Balance) trial assessing Tryngolza (Q4W) vs PBO, which showed reduced triglyceride levels at 6mos., sustained through 12mos., with decrease in acute pancreatitis events over 12mos.; data was published in The NEJM
- Tryngolza is also being studied in a P-III trial among pts with sHTG & triglyceride levels ≥500 mg/dL, with data expected to be released by Sep 2025
Company: Eli Lilly
Product: Kisunla
Active Ingredient: Donanemab
Disease: Symptomatic Alzheimer’s Disease
Date: Sep 25, 2025
Shots:
- The EC has approved Kisunla to treat early symptomatic Alzheimer’s disease in adults with mild impairment or dementia & confirmed amyloid pathology who are ApoE4 heterozygotes or non-carriers
- Approval was based on P-III (TRAILBLAZER-ALZ 2) study (n=1736, ≥18mos.) & P-IIIb (TRAILBLAZER-ALZ 6) trial (n=843, 60-85yrs.) assessing Kisunla (QM) vs PBO in AD pts
- The TRAILBLAZER-ALZ 2 trial showed Kisunla slowed cognitive & functional decline, while TRAILBLAZER-ALZ 6 supported a gradual titration dosing that reduced ARIA-E at 24/52wks. yet maintained comparable amyloid plaque removal & P-tau217 reduction
Note: The following drug has been approved; however, no PR was available:
- Kyinsu (insulin icodec / semaglutide)
Related Post: Insights+: EMA Marketing Authorization of New Drugs in August 2025





