The US FDA New Drug Approvals in September 2025
Shots:
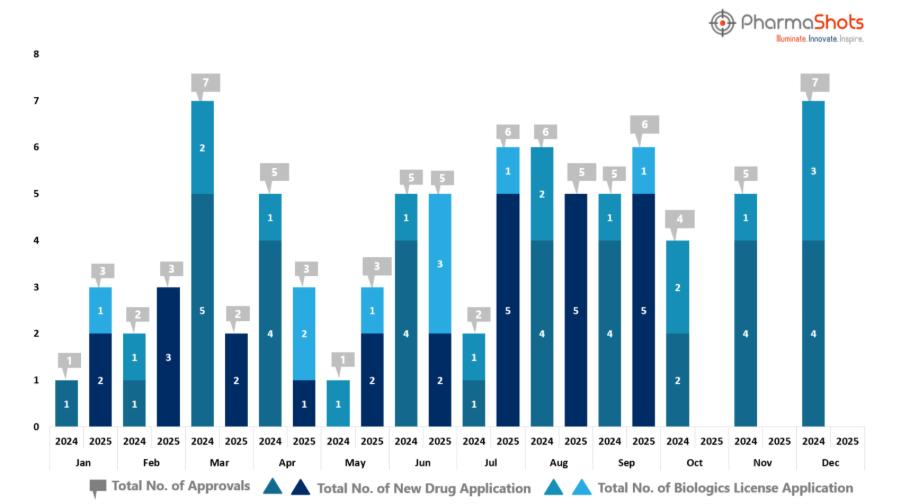
- PharmaShots has compiled a list of US FDA-approved drugs in the month of September 2025
- The US FDA has approved a total of 6 new drugs, including 5 new molecular entities and 1 biologic, leading to the treatment of patients and advances in the pharmaceutical industry
- The major highlighted drug was Merck’s Keytruda Qlex, securing FDA approval Across 38 Solid Tumor Indications for Keytruda
Company: Johnson & Johnson
Product: Inlexzo (drug-device combination)
Active Ingredient: Gemcitabine
Disease: High-Risk Non-Muscle-Invasive Bladder Cancer
Date: Sep 09, 2025
Shots:
- The US FDA has approved Inlexzo (drug-device combination) for the treatment of Bacillus Calmette-Guérin (BCG)-unresponsive HR-NMIBC with CIS &/or papillary tumors
- Approval was based on the P-IIb (SunRISe-1) trial, assessing Inlexzo in pts ineligible or opting out of radical cystectomy, which showed an 82% CR rate, with 51% remaining cancer-free for at least 1yr. post-response
- Inlexzo is an intravesical drug-releasing system that is responsible for the sustained release of gemcitabine into the bladder & is also being evaluated in SunRISe-3 & SunRISe-5 for NMIBC as well as SunRISe-4 for MIBC
Company: Stealth BioTherapeutics
Product: Forzinity
Active Ingredient: Elamipretide HCL
Disease: Barth Syndrome
Date: Sep 19, 2025
Shots:
- The US FDA has received accelerated approval from the US FDA for Forzinity (elamipretide HCl)] to improve muscle strength in pts with Barth syndrome (Wt.≥30 kg or 66 lbs)
- Approval was based on efficacy and safety data from the TAZPOWER trial, which showed improved knee muscle strength
- Forzinity is a mitochondrial cardiolipin binder. It has received multiple FDA designations ODD, FTD, Priority Review, and RPDD as well as ODD status from the EMA. With approval, Stealth also received a Rare Pediatric Disease Priority Review Voucher
Company: Merck
Product: Keytruda Qlex
Active Ingredient: Pembrolizumab & Berahyaluronidase Alfa-pmph
Disease: Across 38 Solid Tumor Indications for Keytruda
Date: Sep 19, 2025
Shots:
- The US FDA has approved Keytruda Qlex (pembrolizumab & berahyaluronidase alfa-pmph) for subcutaneous dosing in adults across 38 approved indications of Keytruda, with US availability expected by late Sep 2025
- Approval was based on P-III (3475A-D77) trial assessing Keytruda Qlex (790mg/9600 units, Q6W) + Pt doublet CT vs IV Keytruda (400mg, Q6W) + Pt doublet CT in 1L metastatic NSCLC adults (n=377) with no EGFR, ALK or ROS1 mutations
- Trial showed that Keytruda Qlex is noninferior to Keytruda in AUC exposure & Ctrough, plus achieved ORR of 45% vs 42%, with comparable PFS & OS observed between the two
Company: Crinetics Pharmaceuticals
Product: Palsonify
Active Ingredient: Paltusotine
Disease: Acromegaly
Date: Sep 25, 2025
Shots:
- The US FDA has approved Palsonify for 1L treatment of adults with acromegaly who are ineligible for or inadequately respond to surgery; commercially available in the US by Oct 2025 & MAA under EMA’s review, with CHMP opinion expected in H1’26
- Approval was based on 2 P-III (PATHFNDR-2 & PATHFNDR-1) trials assessing Palsonify vs PBO in treatment-naïve & experienced pts, respectively, showing rapid, sustained biochemical control with symptom reduction per ASD; OLE data at ENDO’25 showed durable IGF-1 control & symptom relief
- Palsonify (PO, QD), a SST2 nonpeptide agonist, is advancing into P-III (CAREFNDR) trial for carcinoid syndrome, with global enrolment expected throughout 2025
Company: Eli Lilly
Product: Inluriyo
Active Ingredient: Imlunestrant
Disease: Breast Cancer
Date: Sep 25, 2025
Shots:
- FDA approved Inluriyo (200mg; PO) for treating adults with ER+, HER2-, ESR1-mutated advanced or metastatic breast cancer whose disease progressed after ≥1L of endocrine therapy; US availability expected in the coming wks.
- Approval was based on P-III (EMBER-3) trial (N=874: 32% in 1L & 64% in 2L treatment post progression) assessing Inluriyo ± abemaciclib vs fulvestrant/exemestane in ESR1-mutated breast cancer pts (n=256); Inluriyo monotx. showed improved PFS by 38% (mPFS: 5.5 vs 3.8mos.)
- Inluriyo is also being studied in P-III (EMBER-4) trial (with abemaciclib) as an adj. therapy for early breast cancer & P-I (EMBER) trial for advanced or metastatic breast cancer or endometrial cancer
Company: Novartis
Product: Rhapsido
Active Ingredient: Remibrutinib
Disease: Chronic Spontaneous Urticaria
Date: Sep 30, 2025
Shots:
- The US FDA has approved Rhapsido for the treatment of adults with chronic spontaneous urticaria (CSU) who remain symptomatic despite H1 antihistamine treatment. Regulatory filings are also made in the EU, Japan & China, with priority review granted in China
- Approval was backed by P-III (REMIX-1 & 2) trials in CSU, which showed improved itch, hives, & weekly urticaria activity by Wk.12, with more pts achieving well-controlled disease as early as Wk.2 & at Wk.12, while ~33.3% reached complete absence of itch & hives by Wk. 12
- Rhapsido (PO, BD) blocks BTK to inhibit the release of histamine & other proinflammatory mediators for CSU treatment, plus it is being evaluated across multiple trials for CIndU, hidradenitis suppurativa, & food allergy
Related Post: Insights+: The US FDA New Drug Approvals in August 2025





