EMA Marketing Authorization of New Drugs in May 2025
Shots:
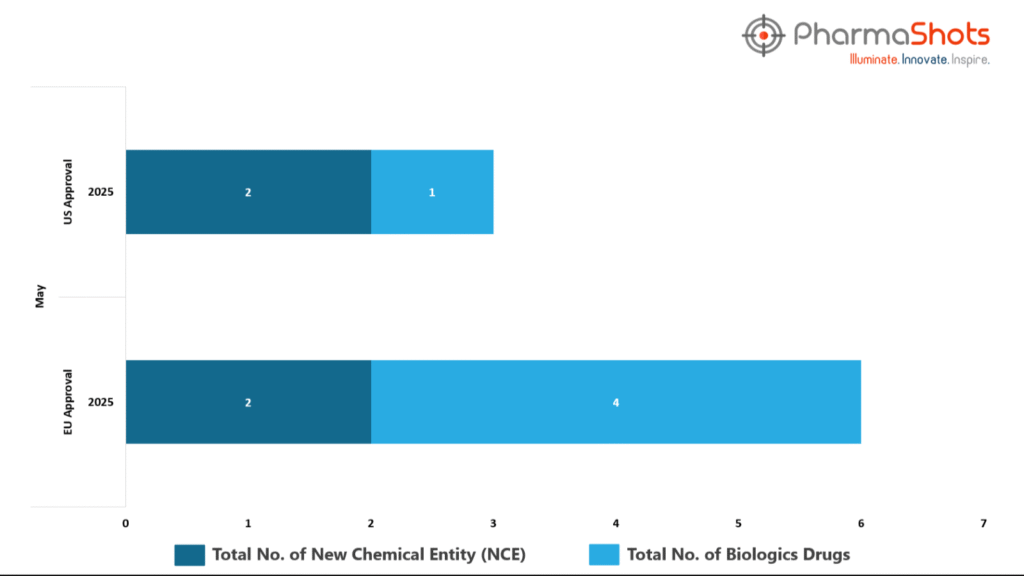
- The EMA’s CHMP has granted positive opinions and approvals to 4 Biologics and 2 New Chemical Entites in May 2025, leading to treatments for patients and advances in the healthcare industry
- The major highlighted drugs were GSk’s Blenrep to Treat R/R Multiple Myeloma
- PharmaShots has compiled a list of 4 drugs that have been granted CHMP’s positive opinions by the EMA, respectively
Company: GSK
Product: Blenrep
Active Ingredient: Belantamab Mafodotin
Disease: R/R Multiple Myeloma
Date: May 22, 2025
Shots:
- CHMP recommended Blenrep + BorDex & PomDex in MM pts with ≥1 prior therapy; EC decision expected in Q3’25. Review ongoing in the US (PDUFA: Jul 23, 2025), Canada, China (PR for DREAMM-7), & Switzerland (PR for DREAMM-8)
- Opinion was based on P-III (DREAMM-7 & DREAMM-8) trials assessing Blenrep + BorDex vs Darzalex + BorDex in 494 pts & Blenrep + PomDex (BPd) vs Velcade + PomDex in 302 pts, respectively
- DREAMM-7 showed improved PFS (1EP; mPFS: 36.6 vs 13.4mos.), depicting a 42% reduced death risk at 39.4 mFU & 3yr. OS rate (2EP) of 74% vs 60%, while DREAMM-8 showed unreached mPFS vs 12.7mos. at 21.8mos. mFU, 71% vs 51% pts alive with no progression at yr. end, favorable OS trend under review, & consistent BPd benefit across all subgroups
Company: SpringWorks Therapeutics
Product: Ezmekly
Active Ingredient: Mirdametinib
Disease: Neurofibromatosis Type 1 Associated Symptomatic Plexiform Neurofibromas
Date: May 22, 2025
Shots:
- The CHMP has recommended conditional approval of mirdametinib for pts (≥2yrs.) with unresectable neurofibromatosis type 1 associated symptomatic plexiform neurofibromas (NF1-PN) based on P-IIb (ReNeu) trial; EC’s decision expected in Q3’25
- The P-IIb (ReNeu) study assessed mirdametinib (2mg/m^2, BID) in 2 Arms (N=114: 56 pediatric & 58 adults) & met its 1EP of cORR (52% & 41%) with durable tumor volume reduction of -42% (Range: -91 to 48%) & -41% (Range: -90 to 13%)
- 90% children & 88% adults had response of at least 12mos., while 48% & 50% had responses for ≥24 mos. Both groups showed early, sustained pain relief & improved quality of life
Company: Roche
Product: Itovebi
Active Ingredient: Inavolisib
Disease: Breast Cancer
Date: May 22, 2025
Shots:
- The CHMP has recommended Itovebi + Ibrance & fulvestrant as a 1L therapy for adults with PIK3CA-mutated, HR+/HER2- locally advanced or metastatic breast cancer recurring on or within 12mos. of adj. endocrine therapy, based on the P-III (INAVO120) trial
- Trial (n=325) assessed the regimen vs PBO + Ibrance & fulvestrant, improved PFS (1EP) by 57% (mPFS: 15 vs 7.3mos.), with consistent benefit across subgroups; a meaningful OS benefit was observed, with full OS data were shared at ASCO 2025
- Additionally, Roche is evaluating Itovebi in 3 more P-III trials (INAVO121, INAVO122 & INAVO123) for the same indication in various combinations
Company: Autolus Therapeutics
Product: Aucatzyl
Active Ingredient: Obecabtagene Autoleucel
Disease: R/R B-Cell Acute Lymphoblastic Leukemia
Date: May 22, 2025
Shots:
- The CHMP has recommended Obecabtagene Autoleucel (obe-cel; autologous CD19 CAR T cell therapy) for treating pts (≥26yrs.) with r/r B-ALL; EC’s decision on conditional MAA is expected within 2mos.
- Opinion was based on the P-Ib/II (FELIX) study assessing obe-cel in over 100 adults with r/r B-cell precursor ALL across 30 sites in the US, UK & EU
- Trial showed that pts in cohort IIA (n=94) receiving ≥1 infusion of obe-cel had a 76.6% CR/CRi rate, with a mDoR of 21.2mos. & mEFS of 11.9mos.; 6 & 12mos. EFS rates were 65.4% & 49.5%, respectively. Data was published in The NEJM
Note: The following drug has been approved; however, no PR was available:
- Deqsiga (human normal immunoglobulin)
The following drug has been recommended for approval; however, no PR was available:
- Maapliv (amino acids)
Related Post: Insights+: EMA Marketing Authorization of New Drugs in April 2025




