The US FDA New Drug Approvals in May 2025
Shots:
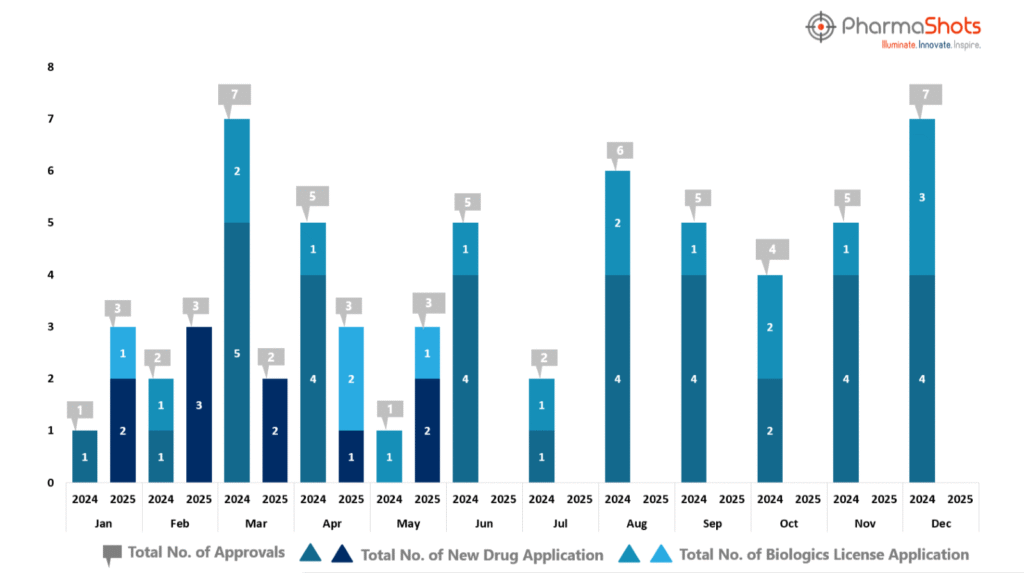
- PharmaShots has compiled a list of US FDA-approved drugs in the month of May 2025
- The US FDA has approved a total of 3 new drugs, including 2 new molecular entities and 1 Biologic leading to the treatment of patients and advances in the healthcare industry
- The major highlighted drug was AbbVie’s Emrelis securing FDA approval for treating NSCLC With High c-Met Protein Overexpression
Company: Verastem Oncology
Product: Avmapki + Fakzynja
Active Ingredient: Avutometinib + Defactinib
Disease: KRAS-Mutated Low-Grade Serous Ovarian Cancer
Date: May 08, 2025
Shots:
- This accelerated approval for the combination of Avmapki (avutometinib) + Fakzynja (defactinib) was granted in advance to the planned PDUFA of Jun 30, 2025, which will be available in the US within a week as a co-pack
- Approval was based on P-II (RAMP 201) trial assessing Avmapki (3.2mg, twice weekly) + Fakzynja (200 mg, BID) in the above pts (n=57) dosed for first 3wks. of 4wk. cycle, which showed cORR of 44% per BICR & mDoR ranging from 3.3 to 31.1mos.
- Regimen is being assessed in P-III (RAMP 301) trial (vs CT) for LGSOC ± KRAS mutation & P-Ib/II (RAMP 205) trial with CT for 1L metastatic pancreatic cancer; P-I/II (RAMP 203) trial assessed the regimen ± defactinib in therapy-naïve & experienced KRAS G12C mutant NSCLC
Company: AbbVie
Product: Emrelis
Active Ingredient: Telisotuzumab vedotin-tllv
Disease: NSCLC with High c-Met Protein Overexpression
Date: May 14, 2025
Shots:
- The US FDA has granted accelerated approval to Emrelis (telisotuzumab vedotin-tllv) for treatment-experienced pts with locally advanced or metastatic, c-Met overexpressing, non-squamous NSCLC
- Approval was backed by an ongoing P-II (LUMINOSITY) study assessing Emrelis as 2L/3L treatment of c-Met overexpressing NSCLC; showing an ORR of 35% & mDoR of 7.2mos. in pts (n=84) with high c-Met protein overexpression
- Emrelis is also being evaluated as a monotx. in P-III (TeliMET NSCLC-01) confirmatory trial for previously treated c-Met overexpressing NSCLC, with global enrollment underway
Company: Alcon
Product: Tryptyr
Active Ingredient: Acoltremon
Disease: Dry Eye Disease
Date: May 28, 2025
Shots:
- The US FDA has approved Tryptyr (0.003% acoltremon ophthalmic solution; AR-15512) to treat signs & symptoms of dry eye disease (DED); US launch expected in Q3’25
- Approval was backed by 2 P-III (COMET-2 & COMET-3) trials assessing Tryptyr vs vehicle in >930 pts with DED, where Tryptyr showed ≥10mm natural tear production increase by Day 14 (42.6% vs 8.2% and 53.2% vs 14.4%), with effects seen as early as Day 1 & sustained through Day 90
- In preclinical studies, acoltremon was shown to activate TRPM8 thermoreceptors, triggering trigeminal nerve pathways associated with increased basal tear production, though Tryptyr’s exact MoA in DED remains unclear
Related Post: Insights+: The US FDA New Drug Approvals in April 2025




