PharmaShots Weekly Snapshots (Jun 02, 2025 – Jun 06, 2025)
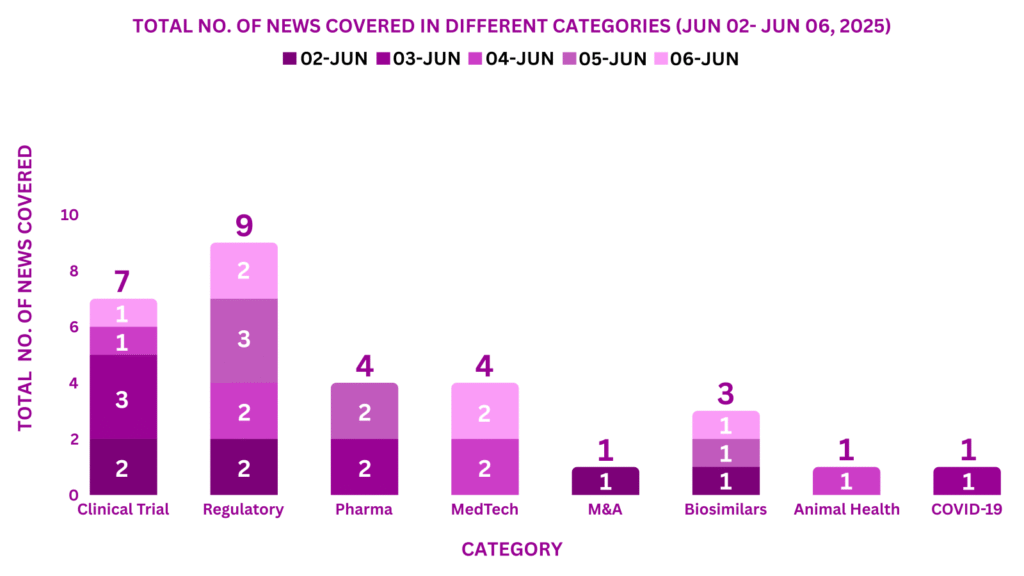
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, Biosimilar, COVID-19 & Animal Health. Check out our full report below:

AstraZeneca Presents P-III (MATTERHORN) Trial Data of Imfinzi as Perioperative Therapy for G/GEJ Cancers at ASCO 2025
Read More: AstraZeneca
Novartis Reveals Interim P-III (PSMAddition) Trial Data of Pluvicto for Metastatic Hormone-Sensitive Prostate Cancer (mHSPC)
Read More: Novartis
Amgen Highlights P-III (DeLLphi-304) Trial Findings of Imdelltra for SCLC at ASCO 2025
Read More: Amgen
Bayer Presents P-III (OASIS 4) Study Data of Elinzanetant for Vasomotor Symptoms (VMS) Associated with Breast Cancer Therapies at ASCO 2025
Read More: Bayer
Roche Reports P-III (IMforte) Trial Findings on Tecentriq Regimen as a 1L Maintenance Therapy for ES-SCLC
Read More: Roche
J&J Presents P-III (AMPLITUDE) Trial Data of Akeega for Metastatic Castration-Sensitive Prostate Cancer (mCSPC) at ASCO 2025
Read More: J&J
Otsuka Pharmaceutical Reports Interim P-III (VISIONARY) Trial Data of Sibeprenlimab for Immunoglobulin A Nephropathy
Read More: Otsuka Pharmaceutical

Kura Oncology and Kyowa Kirin Report the US FDA’s NDA Acceptance and Priority Review of Ziftomenib for Acute Myeloid Leukemia
Read More: Kura Oncology and Kyowa Kirin
GSK Reports the US FDA’s NDA Acceptance of Linerixibat for Primary Biliary Cholangitis (PBC) Patients with Cholestatic Pruritus
Read More: GSK
Bayer Reports the US FDA’s Approval of Nubeqa for Treating Metastatic Hormone-Sensitive Prostate Cancer (mHSPC)
Read More: Bayer
Takeda’s Adcetris Regimen Receives the EC’s Approval to Treat Newly Diagnosed Hodgkin Lymphoma
Read More: Takeda
Akeso’s Cadonilimab Receives the NMPA’s Approval for 1L Treatment of Cervical Cancer
Read More: Akeso
HUTCHMED and Innovent Biologics Report NMPA’s NDA Acceptance of Fruquintinib + Sintilimab for Renal Cell Carcinoma
Read More: HUTCHMED and Innovent
The EC Approves Roche’s Evrysdi Tablet for Spinal Muscular Atrophy
Read More: Roche
AstraZeneca’s Calquence Regimen Receives the EC’s Approval for 1L Chronic Lymphocytic Leukaemia (CLL)
Read More: AstraZeneca
Sydnexis’ SYD-101 Receives the EC’s Approval to Reduce Pediatric Myopia Progression
Read More: Sydnexis

BMS Enters a ~$11.1B Deal with BioNTech to Jointly Develop and Commercialize BNT327
Read More: BMS and BioNTech
Regeneron Enters a ~$2B In-Licensing Deal with Hansoh Pharma for HS-20094, Expanding its Obesity Portfolio
Read More: Regeneron and Hansoh Pharma
Eli Lilly Licenses Camurus’ FluidCrystal Technology to Advance Long-Acting Incretin Products
Read More: Eli Lilly and Camurus
Cullinan Therapeutics Enters a ~$712M Licensing Deal with Genrix Bio for Velinotamig
Read More: Cullinan Therapeutics and Genrix Bio

Amber Implants Reports Trial Data of VCFix Spinal System for Treating Vertebral Compression Fractures
Read More: Amber Implants
TELA Bio Launches OviTex Inguinal for Robotic and Laparoscopic Inguinal Hernia Repair in the EU
Read More: TELA Bio
Avicenna.AI Receives European CE Mark Approval for its CINA-VCF Quantix and CINA-CSpine
Read More: Avicenna.AI
Tracer Biotechnologies Collaborates with QIAGEN to Co-Develop and Commercialize Tracer dPCR & Tracer WGS for Solid Tumors
Read More: Tracer Biotechnologies and QIAGEN

Sanofi to Acquire Blueprint Medicines for ~$9.5B
Read More: Sanofi and Blueprint Medicines

Sandoz Launches Wyost and Jubbonti (Biosimilars, Xgeva and Prolia) in the US
Read More: Sandoz
Alvotech Collaborates with Dr. Reddy’s to Co-Develop a Biosimilar Version of Keytruda
Read More: Alvotech & Dr. Reddy’s
Formycon Receives the ANVISA Approval for Ranivisio (Biosimilar, Lucentis) Across Brazil
Read More: Formycon

Zoetis Launches AI Masses to Detect Skin and Lymph Lesions
Read More: Zoetis

Moderna’s mNEXSPIKE Receives the US FDA’s Approval to Protect Against COVID-19
Read More: Moderna
Related Post: PharmaShots Weekly Snapshots (May 26, 2025 – May 30, 2025)

