BioVentrix's Revivent TC Transcatheter Ventricular Enhancement System Receives FDA's Breakthrough Device Designation for Heart Failure
Shots:
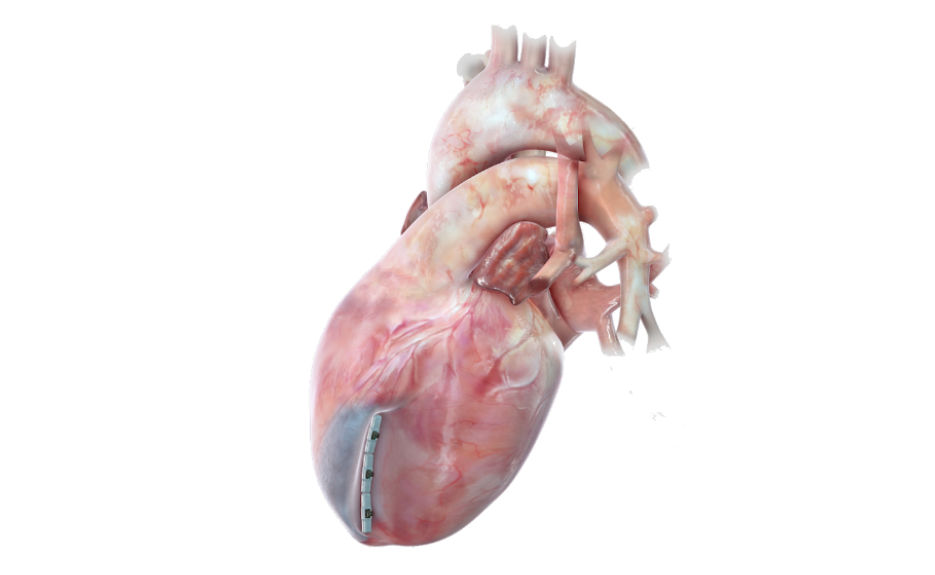
- The US FDA has granted breakthrough device designation to the Revivent TC system using in LIVE procedures for excluding scar tissue on the left ventricle- resulting from a heart attack- allowing a healthy portion of the heart to function efficiently
- The LIVE procedure involves the implantation of anchor pairs on the scar along the septum of the right ventricle and outer surface of the left ventricle- leading to remodeling of the heart to a more normal shape and size and reduces wall stress- thus improves blood flow throughout the body. LIVE process remodels the heart- regain its function & renew life
- Revivent TC System is less invasive therapy for the patients with ischemic HF- providing relief by reducing the volume of abnormally dilated heart and increases ejection fraction- will evaluate in an ALIVE study enrolling ~120 patients across the US. Revivent TC System has received CE mark & is commercially available in the EU
Click here to read full press release/ article | Ref: BioVentrix | Image: Cath Lab Digest

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com