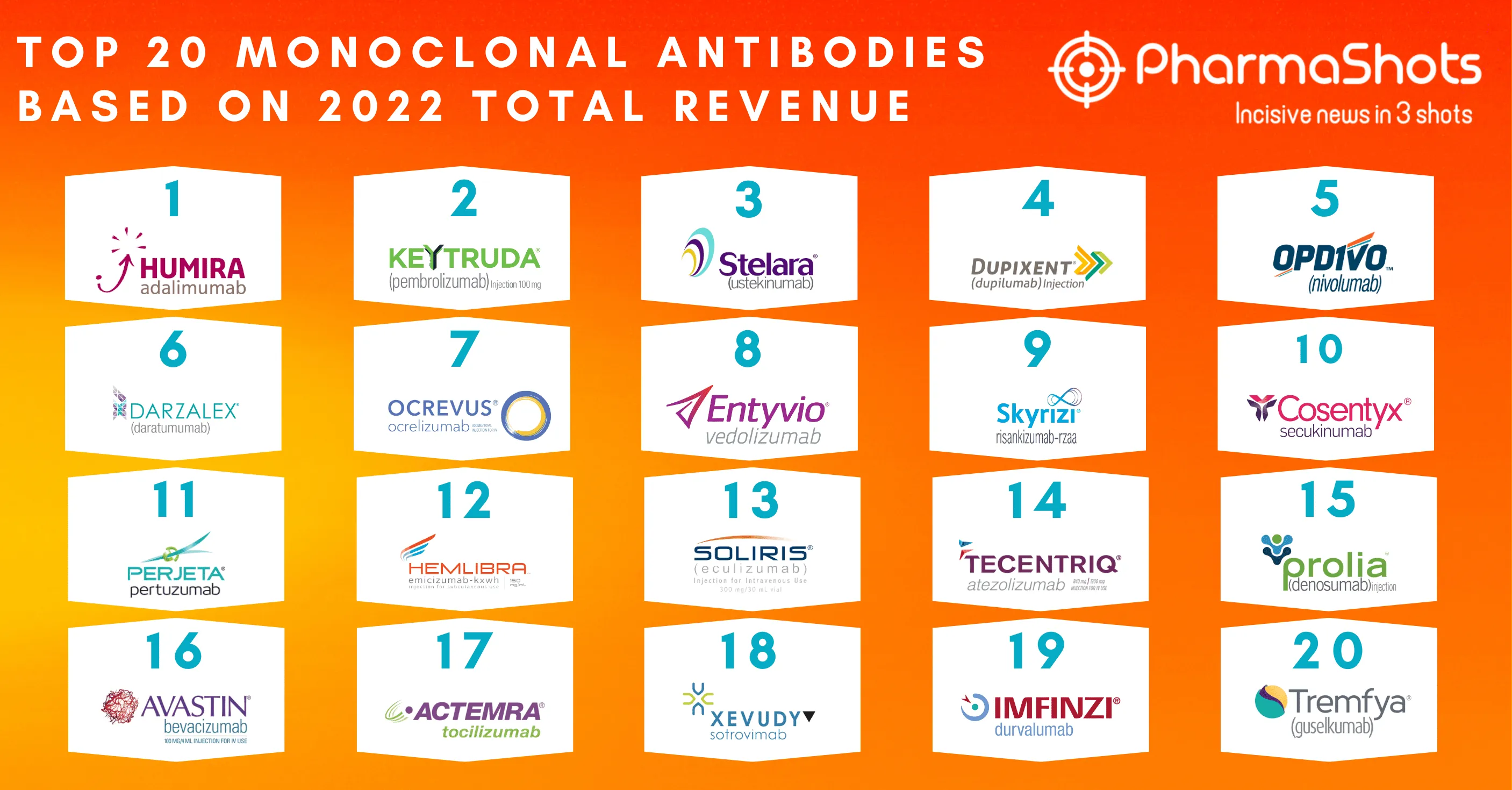
Bayer and Janssen's Xarelto (rivaroxaban) Receive FDA's Approval to Prevent Venous Thromboembolism in Acutely Ill Patients
Shots:
- The approval is based on P-III MAGELLAN study assessing Xarelto in preventing VTE in patients with acute medical illnesses- at risk for thromboembolic complications who are not at high risk of bleeding and is supported by P-III MARINER study assessing Xarelto vs PBO to prevent VTE and VTE-related death following hospital discharge
- The P-III MAGELLAN study resulted in meeting its two 1EPs i.e- non-inferiority to enoxaparin in short-term use (10 ± 4 days) and superiority in long-term use (35 ± 4 days) compared to short-term use of enoxaparin followed by PBO
- Xarelto is the only non-vitamin K antagonist oral anticoagulant (NOAC)- discovered by Bayer- being jointly developed with Janssen and is approved in the US for the continuum of VTE care- from prevention and treatment of initial VTE via extended prevention of recurrent VTE
Click here to read full press release/ article
Ref: Bayer | Image: Janssen

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com