A Complete Account of FDA Approvals in 2024
Shots:
- In 2024, around 50 new drugs were approved by the US FDA across several indications
- PharmaShots, in an enlightening report, brings a summarized analysis of the approved drugs. The most explored section remains Oncology, Hematology, Dermatology, and Cardiology
- For the complete report with analysis, reach out to us at connect@pharmashots.com
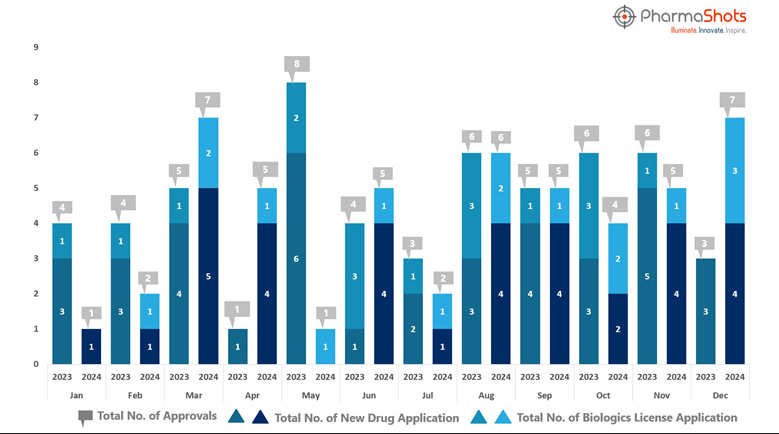
While you embraced the new year delights, PharmaShots was busy compiling a detailed analysis of the list of drugs approved in 2024. By analyzing drugs and indications visually, you can get a more comprehensive view of therapy areas on which biopharma companies are focusing.

Oncology:
Oncology remains the most explored domain of therapy areas in 2024. In, 2024 witnessed 15 noteworthy approvals. Johnson & Johnson’s Rybrevant (amivantamab-vmjw) + Lazcluze (lazertinib) was approved in the US as a 1L treatment for LA/metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations. Imdelltra (Tarlatamab-dlle) from Amgen is indicated for treating adult patients with ES-SCLC with disease progression on or after pt-based CT.
BeiGene’s Tevimbra (tislelizumab-jsgr) is a PD-1 receptor with the US FDA’s approval to treat unresectable or metastatic ESCC after previous systemic CT excl. PD-(L)1 inhibitor. Ensacove (ensartinib) by Xcovery Holdings is a kinase inhibitor indicated for treating adult patients with (ALK-positive locally advanced or metastatic NSCLC who have not previously received an ALK inhibitor.
Hematology:
2024 saw 6 remarkable approvals for several hematology indications. Pfizer’s Hympavzi (marstacimab-hncq ) is a TFPI antagonist as a prophylactic treatment to prevent bleeding episodes in patients (≥12yrs.) with hemophilia A & B without FVIII & FIX inhibitors, respectively.
The US FDA approved Ionis Pharmaceuticals’ Tryngolza (olezarsen), an APOC-III-directed antisense oligonucleotide (ASO), as an adj. for reducing triglycerides (TG) in FCS patients. Piasky (crovalimab-akkz) is a complement C5 inhibitor by Roche, approved for the treatment of patients (age≥ 13yrs.; body wt.≥40kg) with paroxysmal nocturnal hemoglobinuria (PNH)
Vafseo (vadadustat) is a HIF PH inhibitor by Akebia Therapeutics, approved for treating anemia associated with chronic kidney disease (CKD) in adults on dialysis for at least 3mos.

The report only covers the highlights from Oncology and Hematology. For a complete report, reach out to us at connect@pharmashots.com
Closing Note: Driven by innovation, biopharma companies are forging ahead at a momentous pace. The approval of each drug represents a small victory and holds the potential to transform the healthcare industry in its own way.
Related Post: A Complete Account of FDA Approvals in 2023





