New Drug Designations – March 2025
Shots:
- PharmaShots’ Designation Report provides a concise overview of the latest drug designations granted by major regulatory authorities, including the FDA, EMA, MHLW, and NMPA
- The March 2025 report covers designations granted to 37 drugs and 3 medical devices, spanning 11 small molecules, 10 biologics, 10 cell and gene therapies & 3 medical devices among others
- Significant trends this month show, Bioxodes received ODD for hemorrhagic stroke from both the US FDA and EMA
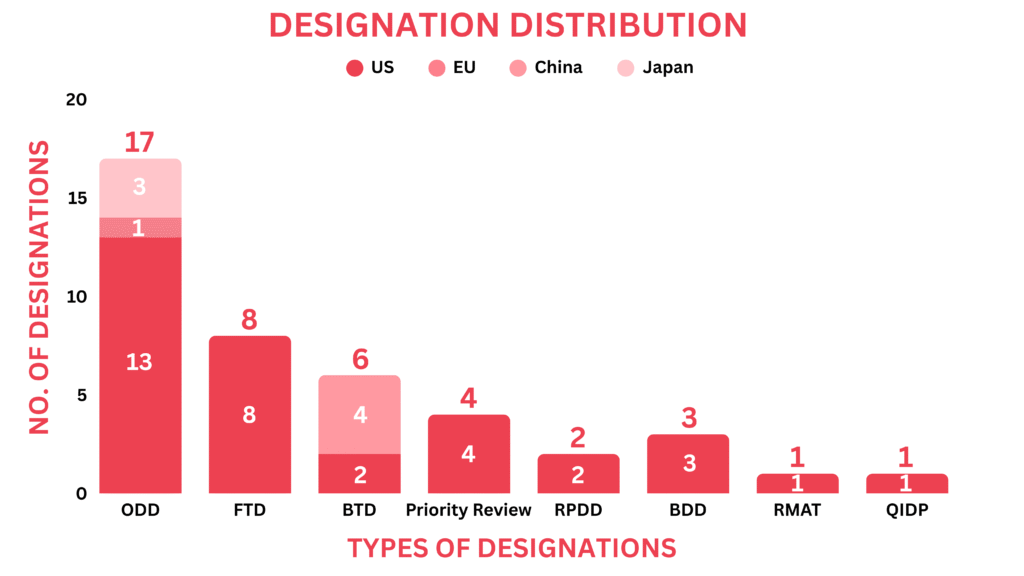

Drugs receiving orphan drug designation by global regulatory bodies echoed as cell & gene therapies, radiopharmaceuticals, small molecules, and biologics. Around 16 drugs received the orphan drug designation across multiple indications
A Quick Look
Cell & Gene Therapies: ART001 (AccurEdit Therapeutics) for ATTR, EN001 (ENcells) for Charcot-Marie-Tooth Disease, NEU-001 (Neurenati Therapeutics) for Hirschsprung Disease, RCT2100 (ReCode Therapeutics) for Cystic Fibrosis, Small Molecules: PL7737 (Palatin) for Obesity caused by LEPR deficiency, Molecular Hydrogen (DiagnaMed) for ALS, Radiprodil (GRIN Therapeutics) for GRIN-related neurodevelopmental disorder, Bexobrutideg (Nurix Therapeutics) for Waldenström Macroglobulinemia, Biologic: Bexmarilimab (Faron Pharmaceuticals) for Myelodysplastic Syndromes, Amlenetug (Lundbeck) for Multiple System Atrophy, HLX22 (Henlius Biotech, AbClon & Alligator Biosciences) for Gastric Cancer, Batoclimab (HanAll Biopharma) for Active Thyroid Eye Disease, Protein: Setmelanotide (Rhythm Pharmaceuticals) for Hypothalamic Obesity, Peptide: PEP-010 (PEP-Therapy) for Pancreatic Cancer, BIOX-101 (Bioxodes) for intracerebral hemorrhagic stroke, Radiopharmaceutical: 186-Rhenium Obisbemeda (Plus Therapeutics) for Leptomeningeal Metastases in patients with Lung Cancer

About 8 drugs received the fast track designation as small molecules, biologics, protein, platelet and cell & gene therapies
A Quick Look
Cell & Gene Therapy: ASTN-201 (Atsena Therapeutics) for X-linked Retinoschisis, Azer-cel (Imugene) for r/r DLBCL, STM-01 (Secretome Therapeutics) for Heart Failure with Preserved Ejection Fraction, Small Molecule: LTG-001 (Latigo Biotherapeutics) for Non-Opioid Treatment of Acute Pain, Biologic: Nipocalimab (J&J) for Sjogren’s Disease, SGC001 (Sungen Biomedical) for Acute Myocardial Infarction, Platelet: CLPH-511 (Cellphire Therapeutics) for Acute Hemorrhage, Vaccine: Chlamydia mRNA Vaccine (Sanofi) for Chlamydia Infection

Six drugs ranging from biologic and small molecule to cell therapy were given breakthrough therapy designation
A Quick Look
Biologic: IBI363 (Innovent Biologics) for Melanoma, JSKN003 (Alphamab) for platinum resistant recurrent epithelial ovarian cancer, IBP-9414 (Infant Bacterial Therapeutics) for Necrotizing Enterocolitis and Sustained Feeding Tolerance, Small Molecule: Olverembatinib (Ascentage Pharma) for Ph+ Acute Lymphoblastic Leukemia, Darovasertib (IDEAYA Biosciences) for Neoadjuvant Uveal Melanoma, Cell Therapy: Satri-cel (CARsgen Therapeutics) for Claudin 18.2+ G/GEJ

Four drugs ranging from small molecule and biologic to cell therapy were given priority review
A Quick Look
Small Molecule: Finerenone (Bayer) for Heart Failure, Tolebrutinib (Sanofi) for Multople Sclerosis, Biologic: Apitegromab (Scholar Rock) for Spinal Muscular Atrophy, Cell Therapy: Deramiocel (Capricor Therapeutics) for Duchenne Muscular Dystrophy

Two drugs received the rare pediatric disease designation by the US FDA
A Quick Look
Gene Therapy: NEU-001 (Neurenati Therapeutics) for Hirschsprung Disease, Small Molecule: NEO100 (NeOnc Technologies) for pediatric type diffuse high-grade gliomas

Three devices received the breakthrough device designation by the US FDA
A Quick Look
Phantom X (Phantom Neuro), a neural interface platform, ReFlow External Ventricular Drains (Anuncial Medical) for Brain Swelling and Elevated Ventricular Drains, PMcardio (Powerful Medical) for the detection of ST-elevation Myocardial Infarction and STEMI equivalents

One gene therapy drug received the regenerative medicine advanced therapy designation
A Quick Look
Gene Therapy: Nexiguran Ziclumeran (Intellia Therapeutics) for ATTR amyloidosis with cardiomyopathy (ATTR-CM)

One drug received the Qualified Infectious Disease Product designation as a small molecule
A Quick Look
Small Molecule: ALX1 (Vast Therapeutics) for chronic Pseudomonas Infections
For the complete report, reach out to us at connect@pharmashots.com




