
PharmaShots Weekly Snapshots (September 23 – September 27, 2024)
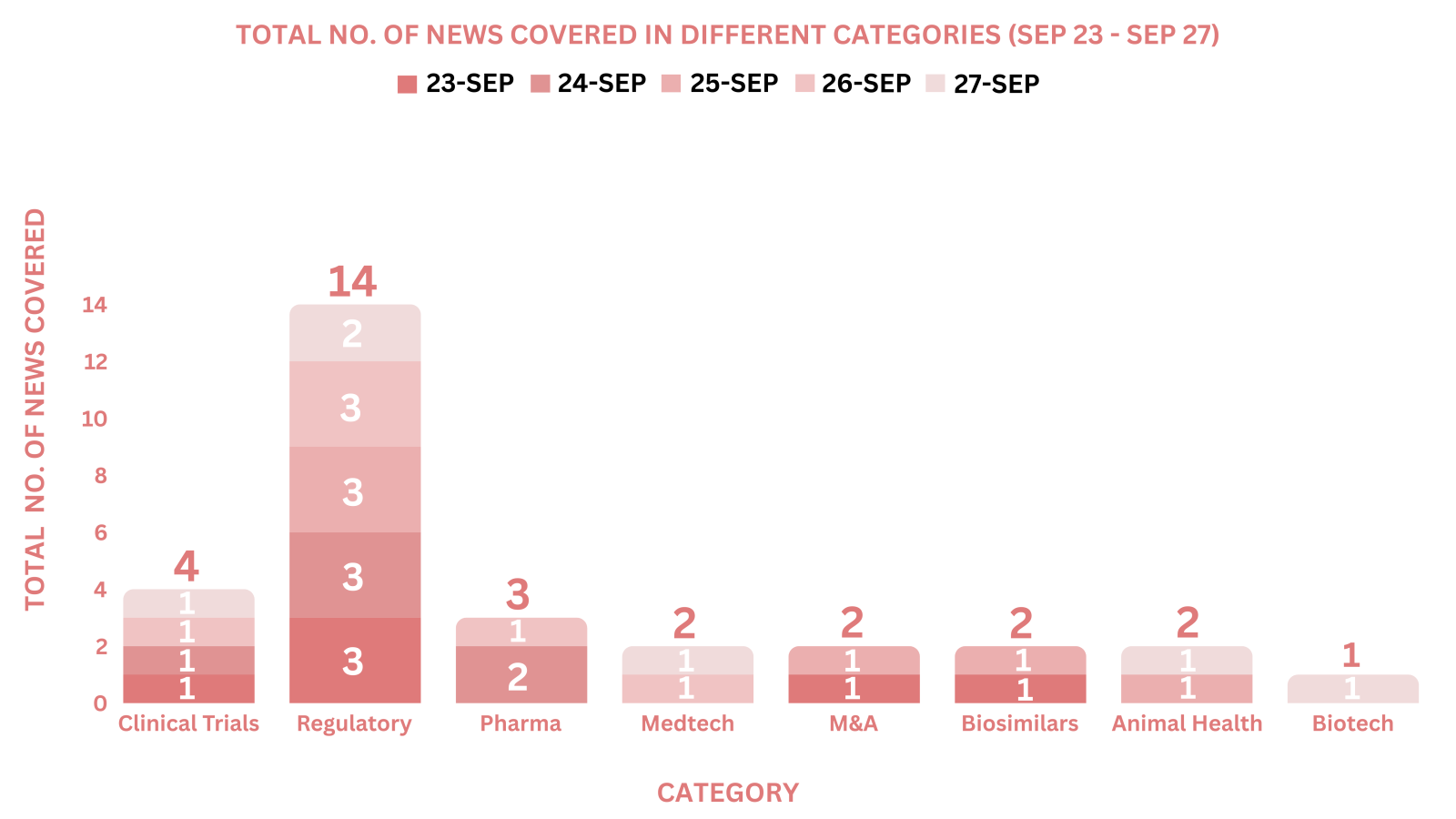
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, Biosimilars, Animal Health & Biotech. Check out our full report below:

AstraZeneca and Daiichi Sankyo Provide Update on the P-III (TROPION-Breast01) Study of Datopotamab Deruxtecan to Treat Breast Cancer
Read More: AstraZeneca & Daiichi Sankyo
Aviceda Therapeutics Concludes Patient Recruitment in P-IIb (SIGLEC) Trial of AVD-104 for Geographic Atrophy Secondary to AMD
Read More: Aviceda Therapeutics
Roche Reports Topline Data from P-III (REGENCY) Trial of Gazyva/Gazyvaro (Obinutuzumab) for Treating Lupus Nephritis
Read More: Roche
AbbVie Reports Topline Data from P-III (TEMPO-1) Study of Tavapadon Alone for Parkinson's Disease
Read More: AbbVie

Merck Reports the CHMP’s Positive Opinions of Keytruda Regimens for Treating Indications of Gynecologic Cancers
Read More: Merck
AbbVie Reports the CHMP’s Positive Opinion of Elahere (Mirvetuximab Soravtansine) to Treat Ovarian Cancer
Read More: AbbVie
Sanofi and Regeneron’s Dupixent Receives the CHMP’s Positive Opinion for Treating Eosinophilic Esophagitis (EoE) in Children
Read More: Sanofi & Regeneron
AstraZeneca’s Fasenra Receives the CHMP’s Positive Opinion for Treating Eosinophilic Granulomatosis with Polyangiitis
Read More: AstraZeneca
GSK Reports the CHMP’s Positive Opinion of Menveo Vaccine Against Invasive Meningococcal Disease
Read More: GSK
Astellas’ Vyloy (Zolbetuximab) Plus Chemotherapy Gains the EC’s Approval for Advanced Gastric and Gastroesophageal Junction Cancer
Read More: Astellas
Takeda Reports the MHLW’s Approval of Fruzaqla (Fruquintinib) to Treat Unresectable Advanced or Recurrent Colorectal Cancer (CRC)
Read More: Takeda
Eli Lilly's Kisunla (Donanemab-azbt) Receives the MHLW’s Approval for Treating Early Symptomatic Alzheimer's Disease (AD)
Read More: Eli Lilly
Junshi Biosciences’ Loqtorzi (Toripalimab) Receives the EC’s Approval for Treating Nasopharyngeal Carcinoma (NPC) and Esophageal Squamous Cell Carcinoma (ESCC)
Read More: Junshi Biosciences
AstraZeneca’s Tagrisso Receives the US FDA’s Approval for Treating Unresectable, Stage III EGFR-Mutated Lung Cancer
Read More: AstraZeneca
IntraBio’s Aqneursa Receives the US FDA’s Approval for Treating Niemann-Pick Disease Type C
Read More: IntraBio
Bayer Reports Regulatory Submission of Nubeqa to the US FDA for Treating Metastatic Hormone-Sensitive Prostate Cancer (mHSPC)
Read More: Bayer
BMS’ Cobenfy (Xanomeline and Trospium Chloride) Receives the US FDA Approval for Treating Schizophrenia
Read More: BMS
LEO Pharma Reports the Regulatory Submission of Enstilar to the NMPA for Treating Plaque Psoriasis
Read More: LEO Pharma

Telix to Expand its US Operations Through the Acquisition of RLS Radiopharmacies
Read More: Telix & RLS Radiopharmacies
Trinity Biotech Reports the Acquisition of Metabolomics Diagnostics for $1.3M
Read More: Trinity Biotech & Metabolomics Diagnostics

The CHMP Grants Positive Opinion to Samsung Bioepis and Biogen’s Opuviz (Biosimilar, Eylea)
Read More: Samsung Bioepis & Biogen
Alvotech Reports the Initiation of Confirmatory Trial of AVT16 (Biosimilar, Entyvio)
Read More: Alvotech
BioAtla Join Forces with Context Therapeutics to Develop and Commercialize BA3362 for Cancer Treatment
Read More: BioAtla & Context Therapeutics
Generate:Biomedicines Partners with Novartis for the Discovery of Protein Therapeutics in Various Indications
Read More: Generate:Biomedicines & Novartis
Orion and Aitia Join Forces for AI-Based Drug Discovery and Drug Simulation in Oncology
Read More: Orion & Aitia

Merck Animal Health Expands NOBIVAC NXT Platform to Include Vaccine for Feline Leukemia Virus
Read More: Merck Animal Health
Zoetis Collaborates with Danone to Develop Sustainable Practices in Dairy Farming Using Genetic Innovation
Read More: Zoetis & Danone

Fujirebio Reports the Regulatory Submission of Lumipulse G pTau 217/β-Amyloid 1-42 Plasma Ratio IVD Test to the US FDA for Alzheimer's Disease
Read More: Fujirebio
Establishment Labs Reports the US FDA’s Approval of Motiva Implants
Read More: Establishment Labs

ReCode Therapeutics Reports the Preclinical Data of RCT2100 for Cystic Fibrosis
Read More: ReCode Therapeutics
Related Post: PharmaShots Weekly Snapshots (September 16 – September 20, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














