PharmaShots Weekly Snapshots (August 20 – August 23, 2024)
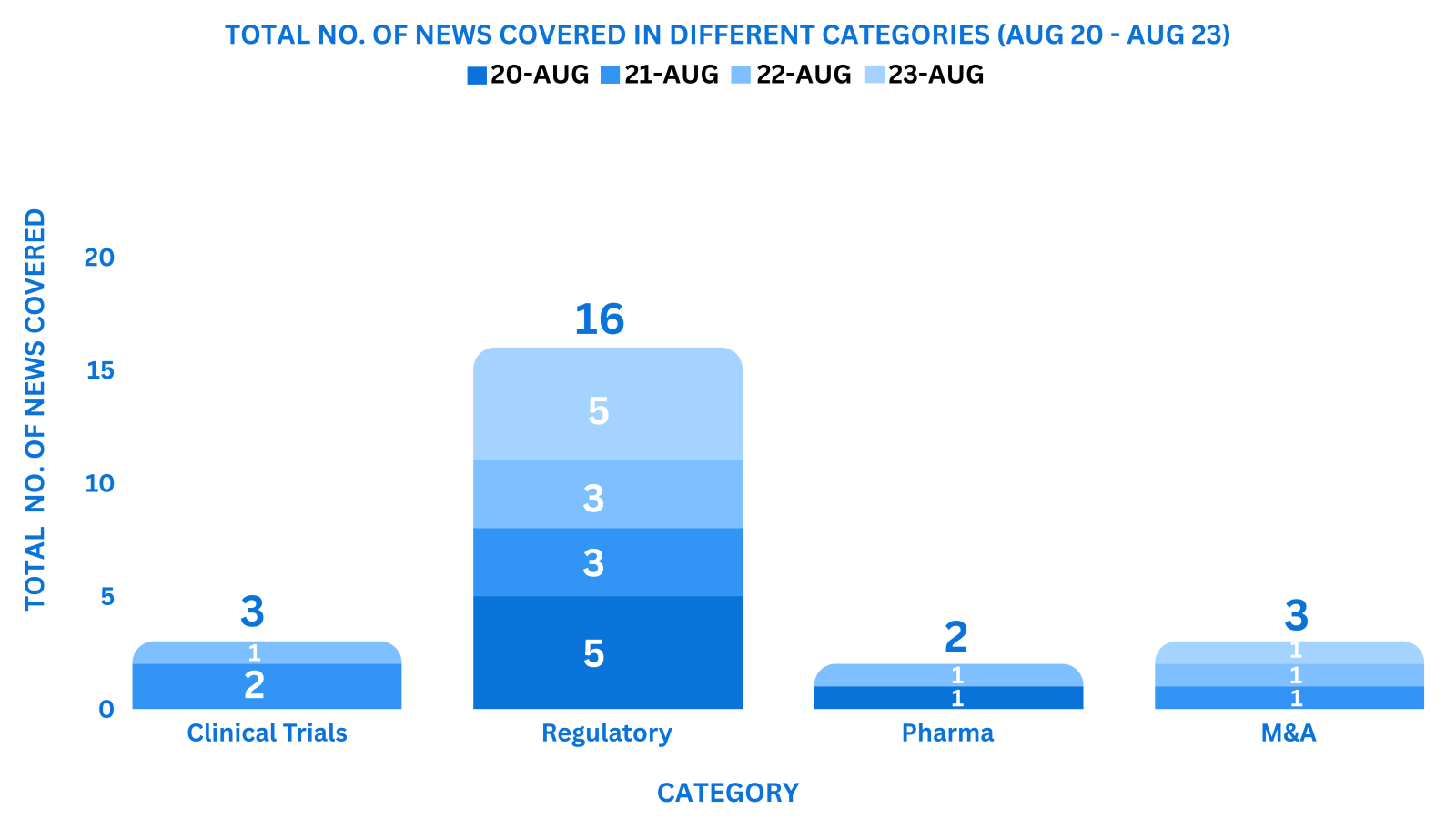
This week PharmaShots’ news was all about the updates on Clinical Trials, Pharma, Biotech, COVID-19, Regulatory & MedTech. Check out our full report below:

Eli Lilly Reports Topline Data from P-III (SURMOUNT-1) Trial of Tirzepatide to Treat Type 2 Diabetes
Read More: Eli Lilly
Regeneron Showcases Updated on BLA for Linvoseltamab to Treat R/R Multiple Myeloma (MM)
Read More: Regeneron
Tris Pharma Initiates Pivotal P-III Study to Evaluate Cebranopadol for Moderate-to-Severe Acute Pain
Read More: Tris Pharma

Daiichi Reports the EMA’s Validation of Application for Enhertu to treat HER2 Low or HER2 Ultralow Metastatic Breast Cancer
Read More: Daiichi Sankyo
BioNTech Reports the Lifting of Partial Clinical Hold for BNT326/YL202
Read More: BioNTech
Daiichi Sankyo’ Enhertu Gains the US FDA’s Breakthrough Therapy Designation to Treat HER2 Low or HER2 Ultralow Metastatic Breast Cancer
Read More: Daiichi Sankyo
GSK’s GSK5764227 (GSK’227) Gets the US FDA’s Breakthrough Therapy Designation for r/r Extensive-Stage Small-Cell Lung Cancer (ES-SCLC)
Read More: GSK
The NMPA Approves Astellas’ Padcev (Enfortumab vedotin) to Treat Adults with Advanced or Metastatic Urothelial Cancer
Read More: Astellas
Liquidia Corporation’s Yutrepia Receives the US FDA’s Tentative Approval to Treat PAH & PH-ILD
Read More: Liquidia Corporation
AstraZeneca’s Fasenra Receives NMPA’s Approval to Treat Severe Eosinophilic Asthma
Read More: AstraZeneca
Johnson & Johnson Reports the US FDA’s Approval of Rybrevant + Lazcluze as a 1L Treatment of NSCLC with EGFR Mutations
Read More: Johnson & Johnson
AbbVie Receives EC’s Conditional Marketing for Tepkinly (Epcoritamab) to R/R Follicular Lymphoma (FL)
Read More: AbbVie
The NMPA Approves Innovent Biologics’ Dupert (Fulzerasib) to Treat NSCLC
Read More: Innovent Biologics
BioArctic and Eisai Receives MAA from MHRA for Leqembi (lecanemab) to Treat Early Alzheimer’s Disease (AD) in Great Britain
Read More: BioArctic & Eisai
Ractigen Therapeutics’ RAG-18 Gains the US FDA’s Orphan Drug Designation to Treat DMD and BMD
Read More: Ractigen Therapeutics
ReAlta Life Sciences’ RLS-0071 Gains the US FDA’s ODD & FTD to Treat Steroid-Refractory aGvHD
Read More: ReAlta Life Sciences
Abata Therapeutics' ABA-101 Gets the US FDA FTD to Treat Progressive Multiple Sclerosis (MS)
Read More: Abata Therapeutics
Galapagos Receives the US FDA’s IND Clearance of GLPG5101 to Treat R/R Non-Hodgkin lymphoma (NHL)
Read More: Galapagos

Bio-Thera Solutions Expands its Partnership with Pharmapark for BAT2306 (Biosimilar, Cosentyx) Across Russia and Other Countries
Read More: Bio-Thera Solutions
Amgen’s Otezla (Apremilast) is now Accessible in The US to treat Moderate to Severe Pediatric Plaque Psoriasis
Read More: Amgen
Abbott Updates Heart Failure Management by Excluding Aspirin for Patients with the HeartMate 3 Heart Pump
Read More: Abbott

Johnson and Johnson to Acquire V-Wave, Expanding and Strengthening J&J MedTech Segment
Read More: Johnson & Johnson & V-Wave
BerGenBio and Tempus Collaborate to Advance the Development of STK11m to Treat NSCLC
Read More: BerGenBio & Tempus
Stryker Reports the Acquisition of Vertos Medical, Strengthening its Pain Management Portfolio
Read More: Stryker & Vertos Medical
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.