Insights+: EMA Marketing Authorization of New Drugs in July 2024
Shots:
-
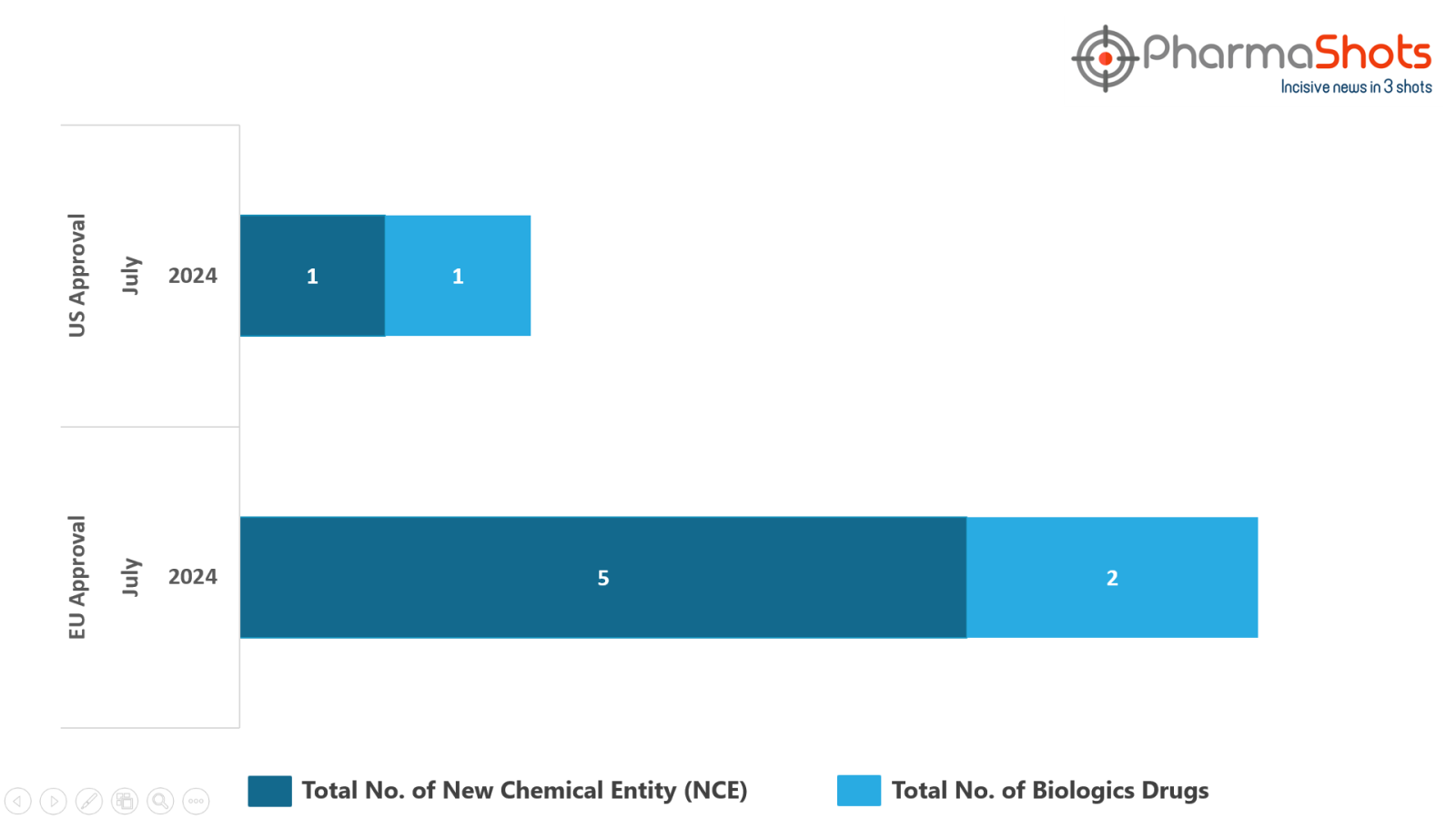
The EMA granted Positive Opinion to 2 Biologics and 5 New Chemical Entities in July 2024, leading to treatments for patients and advances in the healthcare industry
-
The major highlighted drugs were Johnson & Johnson’s Yuvanci to treat Pulmonary Arterial Hypertension (PAH) and Astellas’ Vyloy + CT for Gastric & Gastroesophageal Junction Cancer
-
PharmaShots has compiled a list of 7 drugs that have been granted positive opinion by the EMA’s CHMP
Product Name: Yuvanci
Active ingredient: Combination of Macitentan and Tadalafil
Company: Johnson & Johnson
Date: July 25, 2024
Disease: Pulmonary Arterial Hypertension
Shots:
-
The CHMP has granted positive opinion to Yuvanci [macitentan (10mg) + tadalafil (40mg)] as a substitution therapy of PAH adults, based on P-III (A DUE) study
-
The P-III (A DUE) trial assessed Yuvanci STCT vs macitentan (10mg) & tadalafil (40mg) alone in PAH patients (n=187). Subjects with PVR ≥240 dyn×s/cm5 randomly received M/T STCT (n=108), macitentan alone (n=35) or tadalafil alone (n=44), QD
-
Trial reached its 1EP, showing reduced PVR (as a ratio of wk.16 to baseline) of 29% (effect of 0.71) vs macitentan & 28% (effect of 0.72) vs tadalafil, with similar efficacy observed in subgroups of age, sex, race & baseline WHO FC as well as among treatment-naïve or experienced patients (with ERA or PDE5i)
Product Name: Anzupgo
Active ingredient: Delgocitinib
Company: LEO Pharma
Date: July 25, 2024
Disease: Chronic Hand Eczema
Shots:
-
The CHMP has granted positive opinion to Anzupgo cream (topical pan-JAK inhibitor) for treating moderate to severe chronic hand eczema (CHE), inadequately managed by topical corticosteroids
-
Opinion was based on P-III (DELTA 1 & DELTA 2) trials assessing the safety & efficacy of Anzupgo (BID) vs vehicle for moderate to severe CHE. Those who concluded treatment for 16wks. were rolled over to 36wk. DELTA 3 extension study to assess long-term safety
-
The DELTA 1 & 2 reached their 1EPs & 2EPs, with DELTA 3 trial showing the long-term safety that aligned with prior outcomes. The approval, if granted, would applicable across the whole EU plus Iceland, Norway & Liechtenstein; regulatory filings with other bodies are ongoing
Product Name: Iqirvo
Active ingredient: Elafibranor
Company: Ipsen
Date: July 25, 2024
Disease: Primary Biliary Cholangitis
Shots:
-
The CHMP’s positive opinion of Iqirvo (PPAR agonist) + ursodeoxycholic acid (UDCA) for PBC adults having inadequate UDCA response or Iqirvo alone for UDCA intolerant patients was based on P-III (ELATIVE) study. EC’s decision is anticipated in Q3’24. The company has submitted Iqirvo for PBC to the MHRA
-
The P-III (ELATIVE) study assessed Iqirvo (80mg, QD) vs PBO to treat PBC patients (n=161) having inadequate response or intolerance to UDCA
-
Study reached the composite 1EP of BCR, showing 47% treatment benefit in 51% vs 4% of them at wk.52 with an ALP reduction of 41% in 15% vs 0% of them at wk.4 that was sustained till wk.52
Product Name: Kayfanda
Active ingredient: Odevixibat
Company: Ipsen
Date: July 25, 2024
Disease: Cholestatic Pruritus in Alagille Syndrome
Shots:
-
The CHMP’s positive opinion of Kayfanda to treat cholestatic pruritus in ALGS individuals (≥6mos.) was based on P-III (ASSERT) study. EC’s decision is anticipated in Q3’24
-
The P-III (ASSERT) study assessed the safety & efficacy of Kayfanda (120µg/kg/day) vs PBO for 24wks.to relieve itch in ALGS patients (n=52; 0-17yrs.) across North America, EU, the Middle East & the Asia Pacific regions
-
Study reached its 1EP, showing improved pruritus after 6mos. (90% were itch responders) & 2EP, showing reduction in serum bile acid concentration after 20-24wks. and improved sleep parameters at wks.1-4 that sustained till wk.24
Product Name: Vevizye
Active ingredient: Ciclosporin
Company: Novaliq
Date: July 25, 2024
Disease: Dry Eye Disease
Shots:
-
The CHMP has granted a positive opinion to Vevizye in moderate to severe dry eye disease adults treated with tear substitutes based on (ESSENCE-1 & ESSENCE-2) trials involving >1500 patients
-
Vevizye showed improved ocular surface health, with a reduction in corneal fluorescein staining at D15 (~71.6% responded within 4wks.); additional benefits were observed at up to 56wks., verifying its efficacy & tolerability
-
Vevizye (CyclASol) is a clear, 0.1% ciclosporin solution without oils, surfactants, or preservatives offering superior spreading and longer retention on the eye
Product Name: Vyloy
Active ingredient: Zolbetuximab
Company: Astellas
Date: July 25, 2024
Disease: Gastric and Gastroesophageal Junction Cancer
Shots:
-
The CHMP has granted positive opinion to zolbetuximab + CT, with the decision anticipated in Oct 2024 & applicable across the whole EU plus Iceland, Liechtenstein & Norway. Other worldwide regulatory applications are under review
-
Opinion was supported by 2 P-III studies assessing zolbetuximab plus mFOLFOX6 [SPOTLIGHT (n=565)] & CAPOX [GLOW (n=507)] vs PBO as a 1L treatment of HER2-ve & CLDN18.2+ve G/GEJ adenocarcinoma; published in The Lancet & Nature Medicine, respectively
-
In addition, Astellas & Roche partnered for the VENTANA CLDN18 (43-14A) RxDx Assay, an immunohistochemistry-based companion diagnostic test, to find patients for zolbetuximab treatment. The test’s review is underway by the notified body
Product Name: Loqtorzi
Active ingredient: Toripalimab
Company: Junshi Biosciences
Date: July 25, 2024
Disease: NPC and ESCC
Shots:
-
The CHMP has granted positive opinion to 1L Loqtorzi with cisplatin & gemcitabine for recurrent, metastatic NPC as well as with cisplatin & paclitaxel for unresectable advanced, recurrent or metastatic ESCC; valid across the EU plus Iceland & Norway
-
The opinions for NPC & ESCC were supported by P-III (JUPITER-02 & JUPITER-06) trials, respectively; JUPITER-02 results were highlighted at ASCO 2021 & published in Nature Medicine & JAMA, with JUPITER-06 results featured at ESMO 2021 & published in Cancer Cell & Journal of Clinical Oncology
-
Toripalimab, an anti-PD-1 mAb, works by blocking PD-1 interactions with PD-L1 & PD-L2 ligands to improve endocytosis function
Related Post: Insights+: EMA Marketing Authorization of New Drugs in June 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com