New Drug Designations – December 2023
Shots:
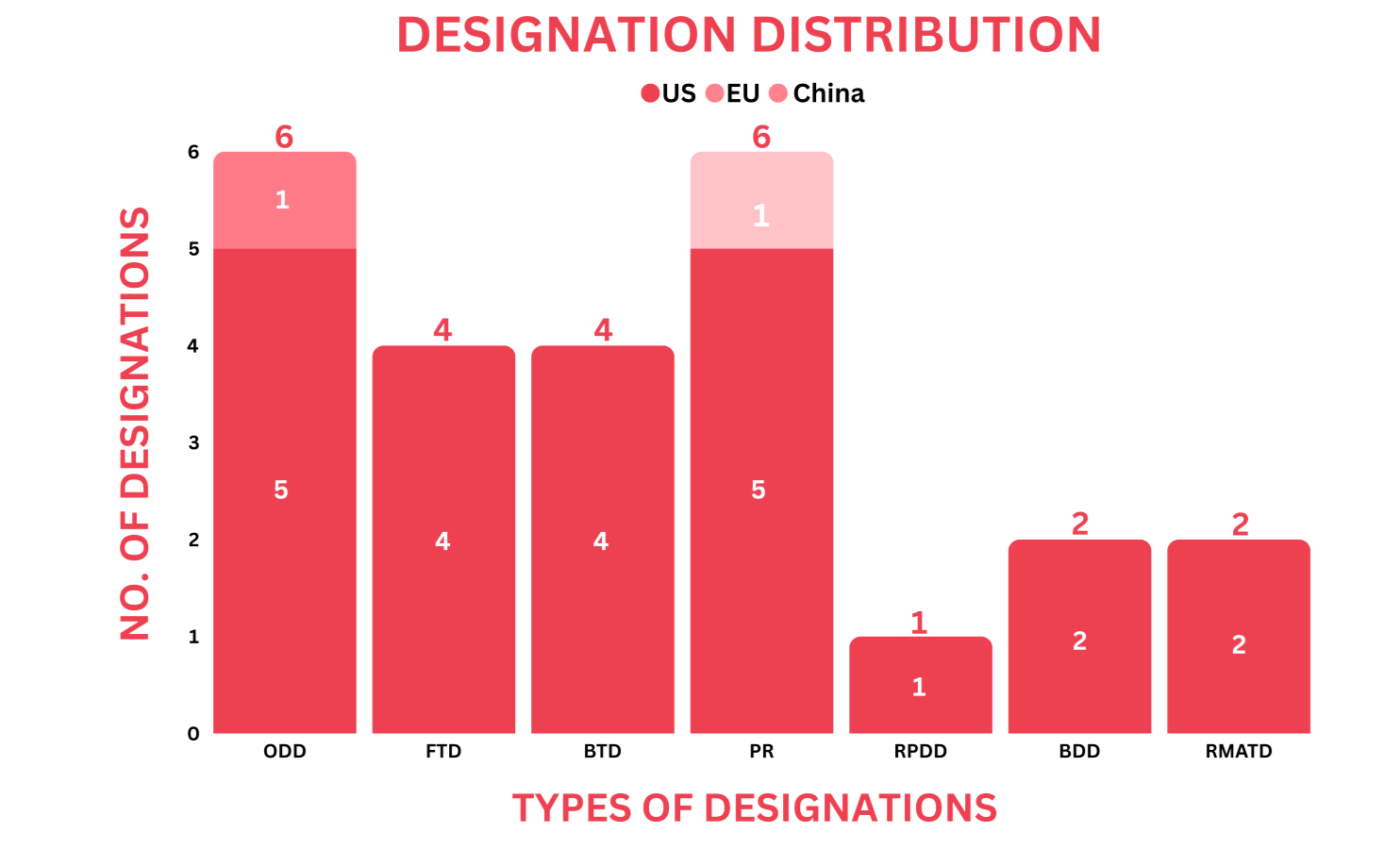
- PharmaShots’ designation report provides a concise overview of several drugs and their designations by the US FDA, EC, and China’s NMPA. This month’s report includes 6 biological drugs, 10 small molecules, 5 cell and gene therapies, 1 recombinant fusion protein, 1 peptide and 2 devices
- Neurocrine Biosciences’ Crinecerfont, focused on the treatment of Congenital Adrenal Hyperplasia (CAH), is the drug to receive BTD and from the US FDA along with previous FTD and RPDD
- PharmaShots has compiled a list of a total of 23 drugs and 2 devices awarded with designations by multiple regulatory bodies in Dec 2023

JR-441

- JR-441 received ODD from the US FDA, an investigational drug for the treatment of MPS IIIA, or Sanfilippo syndrome type A. In Jan 2022, JR-441 received ODD by the EC
- JCR is presently conducting a global P-I/II study for JR-441 in MPS IIIA, with the first patient dosed in Oct 2023
- JR-441 is a form of recombinant heparan N-sulfatase with an ability to penetrate blood-brain barrier (BBB) developed using JCR’s proprietary J-Brain Cargo BBB-penetrating technology
SLS009

- SLS009 has been given ODD by the US FDA for the treatment of r/r PTCL.
- SLS009 is being evaluated in an ongoing open-label, single-arm P-Ib/II study in r/r PTCL patients (n=95). This is expected to be registrational study is fully funded by GenFleet Therapeutics and in China.
- From completed dose-escalation portion of the P-I trial in r/r hematological malignancies. SLS009 showed a favorable safety profile and positive clinical efficacy.
- AML & Lymphoma patients showed Complete or partial responses, incl. four PTCL patients (36.4%), 1 patient showed complete metabolic response and still ongoing for over 62wks. 2nd patient with CR by CT scan continuing treatment for over 24 wks.
- Belinostat (HDACi) presently SoC for r/r PTCL in its pivotal P-II showed 25.8% response rate in similar population
- The P-Ib/II study in PTCL ongoing with top line data expected in 1H’ 2024
NS-089/NCNP-02

- NS Pharma’s NS-089/NCNP-02 received ODD from the European Commission (EC) for the treatment of Duchenne muscular dystrophy on Dec 13, 2023.
- NS-089/NCNP-02 was discovered through combined research of Nippon Shinyaku & the National Center of Neurology and Psychiatry (NCNP). NS-089/NCNP-02 is an antisense nucleic acid which skips a part of the genetic information of the dystrophin gene and produces a functional dystrophin protein with a slightly shorter chain length.
- NS-089/NCNP-2 has also received RPDD in Jun 2023 & BTD, ODD in July 2023 from the US FDA
CLS001

- Cellusion’s CLS001 received ODD from the US FDA to treat bullous keratopathy by meeting specific criteria diagnosis, treatment & prevention of rare disease
- CLS001 is iPS cell-derived corneal endothelial cell substitute and it is alternative cell to corneal endothelial cells
- Cellusion is preparing for clinical study in Japan as well as for a global study
NXL-004

- NXL-004, an experimental world’s first AAV gene therapy product being developed by NeuExcell was awarded ODD by the FDA for the treatment of malignant glioma
- NXL-004 is based on astrocyte-to-neuron (AtN) conversion platform & has shown good efficacy and safety in preclinical trials and in early 2024 will enter the first-in-human study
- The present SoC of GBM incl. surgery, radiotherapy, and CT results in a mOS of merely 15-18 mos. and a 5-year SR below 10% which creates a huge unmet need for treatment
OCE-205

- Ocelot Bio’s OCE-205 received ODD from the US FDA for the treatment of Ascites due to all etiologies except cancer in Dec 2023.
- The company is planning to start OCE-205’s clinical studies in refractory ascites in 2024
- OCE-205 has also received ODD for hepatorenal syndrome in 2022 and Ocelot has completed the enrolment in a P-II study (NCT05309200) for patients with hepatorenal syndrome with acute kidney injury (HRS-AKI)

Cretostimogene Grenadenorepvec

- CG oncology’s Cretostimogene Grenadenorepvec received FTD & BTD from US FDA for High-Risk Bacillus Calmette-Guérin (BCG)-Unresponsive Non-Muscle Invasive Bladder Cancer (NMIBC) with carcinoma in situ with or without Ta or T1 (papillary) tumors
- The results from P-III BOND-003 (NCT04452591) study of cretostimogene grenadenorepvec met its 1EP i.e. CRR: 75.7% and was well tolerated. Interim results as of Oct 5, 2023, from 66 patients were presented at SUO’23
- Cretostimogene grenadenorepvec is being evaluated in an ongoing P-II study (CORE-001) in combination with pembrolizumab for the same indication & also evaluated in an investigator-sponsored study in combination with nivolumab for the treatment of muscle invasive bladder cancer
Naporafenib

- Erasca’s naporafenib in combination with trametinib (Mekinist) received FTD from the US FDA for the treatment of advanced NRAS-mutated unresectable or metastatic melanoma
- Naporafenib is a P-III ready pan-RAF inhibitor with low response rates & mPFS after treatment & in combination with trametinib shows strong & durable anti-tumor activity
- Erasca’s P-III study SEACRAFT-2 which will evaluate the clinical efficacy of naporafenib in combination with trametinib (MEKINIST) vs physician’s choice of therapy in NRAS-mutated metastatic melanoma. SEACRAFT-2 will be expected to start in H1’24
ABSK021

- AbbiskoABSK021 (CSF-1R inhibitor pimicotinib) received FTD from the US FDA for tenosynovial giant cell tumor (“TGCT”). Previously ABSK021has also received BTD & PRIME designation from the US FDA, China NMPA & EMA for TGCT
- The P-III of TGCT is a global study conducted simultaneously in the US, China, Europe & Canada. In P-Ib study an ORR of 87.5% in the 50 mg QD cohort of ABSK021 was demonstrated. This data of study was presented at the 2023 CTOS & P-I dose-escalation trial has been completed in the US
- Abbisko has received an exclusive license from Merck KGaA to commercialize ABSK021 for all indications in Hong Kong, China mainland, Taiwan & Macau. Merck also gave an exclusive global commercialization option, subject to the terms and condition as agreed between both parties
CAN-2409

- Candel Therapeutics’ CAN-2409 plus prodrug (valacyclovir) received FTD from US FDA for Pancreatic ductal adenocarcinoma (PDAC) based on OS results
- In Nov 2023, Candel presented overall data of randomized, P-II study of CAN-2409 + prodrug (valacyclovir or acyclovir) with SoC in patients with borderline resectable PDAC at the SITC
- After receiving 2-3 IV doses of CAN-2409, survival rate of 71.4% at both 24 & 36 mos. post dosing in PDAC patients was observed vs 16.7% SoC arm patients.
- Parallelly, immunological changes observed in the resected pancreatic tissue after CAN-2409 administration suggest activation of effective immunologic antitumoral response in this otherwise “cold” tumor.
- The P-II study was designed to exclusively focused on borderline resectable disease but after a protocol amendment in 2022, the enrollment of patients with locally advanced PDAC was discontinued but study remains active
- After P-Ib study completion, no. of CD8+ tumor infiltration lymphocytes TILs increase at the site of the tumor after CAN-2409 treatment
- The Company’s pivotal P-III study in prostate cancer is being conducted by FDA & Candel will release updated overall survival data in the Q2’24

Crinecerfont

- Neurocrine Biosciences’ crinecerfont received BTD designation from the US FDA for congenital adrenal hyperplasia. Crinecerfont has also received FTD & RPDD for the same indication in 2023
- The safety & efficacy results from the P-III CAHtalyst studies in pediatric & adult patients met 1EPs & 2EPs, showing successful improvement vs SoC in CAH & achieved a >95% completion rate in both studies
- Neurocrine is also planning to submit NDA in 2024 with USFDA
TAR-200

- The US FDA has granted BTD to Johnson & Johnson’s TAR-200 for the treatment of patients with Bacillus Calmette-Guérin (BCG)-unresponsive high-risk non-muscle-invasive bladder cancer (HR-NMIBC)
- BTD was given based on results from SunRISe-1 (NCT04640623), an open-label P-IIb clinical study, where participants were randomized to one of in three cohorts to treat with receive TAR-200 in combination with + cetrelimab (Cohort 1), TAR-200 alone (Cohort 2) or cetrelimab alone (Cohort 3) for BCG-unresponsive HR-NMIBC carcinoma in situ CIS patients
- The 1EPs of the study is Complete Response rate at any time point & 2EPs incl. duration of response DoR, OS, QoL, PK, overall survival, quality of life, PKs, safety & tolerability. Cohorts 1 & 3 were closed to further enrollment effective Jun 1, 2023. Results presented at ESMO’23; AUA’23
VT-X7

- Osteal Therapeutic’s VT-X7 received BTD from the US FDA for the treatment of periprosthetic joint infection (PJI) of the hip & knee. VT-X7 has also received OD, FTD, Priority review & QIDP Designation
- BTD was given based on results from APEX (NCT04662632), a P-IIb, prospective, multi-centre, randomized controlled trial evaluating the safety & efficacy of VT-X7. BTD helps to commercialize the new drug by considering the significant improvement vs SoC
- Osteal Therapeutic also reports completion of enrolment in APEX-2 the pivotal study
BNT323/DB-1303

- BNT323/DB-1303 received BTD from the US FDA for the treatment of advanced endometrial cancer in patients who progressed on after treatment with immune checkpoint inhibitors
- Encouraging results from the ongoing P-I/II study, for which data was also presented at ASCO 2023 and ESGO 2023 demonstrating anti-tumor activity in heavily pretreated patients with advanced endometrial cancer with unconfirmed ORR 58% and DCR 94.1%. Was also found to be well tolerated among advanced solid tumor patients
- In Jan 2023, BNT323/DB-1303 has already received FTD from the FDA for the treatment of endometrial cancer

Elahere (mirvetuximab soravtansine-gynx)

- The US FDA has filed the sBLA based on the P-III (MIRASOL) study of Elahere in platinum-resistant ovarian cancer. The PDUFA assigned date for the application is April 5, 2024, and it has been designated as Priority Review
- Top-line results were disclosed in May 2023. Elahere’s MIRASOL study depicted statistically significant and clinically improvements in PFS, ORR & OS vs low-grade ocular & gastrointestinal events continue to dominate the safety profile of Elahere
- In Nov 2022 Elahere was granted accelerated approval by the FDA. For EU and China approved by EMA and NMPA respectively
Elafibranor

- Genfit & Ipsen’s NDA for elafibranor received priority review from the US FDA for the treatment of primary biliary cholangitis (PBC) & the PDUFA date assigned is Jun’24
- Elafibranor (80mg, QD) is being investigated for its safety and efficacy in the P-III (ELATIVE) study for treating patientswith PBC who are unresponsive or intolerant to ursodeoxycholic acid (UDCA)
- The EMA has also approved MAA for elafibranor, a CHMP review was submitted on Oct 26, 2023 and UK Medicines & MHRA has been validated the 3rd filing for review
Xolair

- Xolair (omalizumab) received priority review from theUS FDA for Children & Adults with food allergies. It was based on NIH’s +ve P-III study interim results. Xolair had also received BTD for the severe allergic reactions in Aug 2018
- The US FDA accepted sBLA based on stage 1 of the NIH-sponsored P-III OUtMATCH (NCT03881696) interim analysis results in patients allergic to peanuts & two other common foods. FDA is expected to make a decision on Xolair in the Q1’24
- Xolair was first approved in 2003 by US FDA for the moderate to severe persistent allergic asthma. It is also approved for chronic spontaneous urticaria (CSU) & chronic rhinosinusitis with nasal polyps (CRSwNP)
Tarlatamab

- The US FDA granted priority review to Amgen’s tarlatamab & accepted BLA for advanced small cell lung cancer (SCLC). BLA is based on the P-II study results from the DeLLphi-301 study & PDUFA date for tarlatamab assigned is Jun 12, 2024
- In the P-I (DeLLphi-300) & P-II (DeLLphi-301) study, tarlatamab showed responses of 23.4% & 40% in patients with advanced SCLC respectively
- In Oct 23, tarlatamab was granted BTD by the FDA and application is being under the Project Orbis framework and Real Time Oncology Review (RTOR).
Patritumab Deruxtecan (HER3-DXd)

- The US FDA has accepted BLA and granted priority review to the Daiichi Sankyo and Merck’s Patritumab Deruxtecan (HER3-DXd) for adult patients with locally advanced or metastatic EGFR-mutated NSCLC & assigned PDUFA date for Jun 26, 2024
- The BLA is based on the P-II (HERTHENA-Lung01) primary study results in which patients (n=225) were randomized 1:1 & received Patritumab Deruxtecan (5.6 mg/kg, IV) once Q3W or an up titration regimen (n=50) received Patritumab Deruxtecan (3.2 → 4.8 → 6.4 mg/kg)
- The study duration was 18.9 mos., ORR (1EP, 29.8%), mDoR (6.4 mos.), mPFS (11.9 mos.) & safety profile was tolerable, manageable & consistent
Taletrectinib

- AnHeart’s partner Innovent Biologics received PRD from CDE of NMPA for the NDA application for Taletrectinib (ROS1- TKI) used for the treatment of NSCLC. CDE accepted the NDA in Nov 2023
- The data from the P-II (TRUST-I) study (NCT04395677) is the basis for the NDA and PRD in China was also presented at ELCC 2023. TRUST-II is a 2nd study for taletrectinib at global level (NCT04395677)
- Taletrectinib was granted BTD by the CDE of China’s NMPA in March 2022 and also received BTD by the US FDA

HG302

- HuidaGene Therapeutics’ HG302, a novel CRISPR-Cas12 DNA-editing therapy, received RPDD from US FDA for the treatment of Duchenne muscular dystrophy (DMD). This is company’s 3rd program which received RPDD
- HuidaGene has developed this molecule using proprietary AI HG-PRECISE platform
- If the HG302 BLA is approved by US FDA for DMD, HuidaGene is expected to be eligible to receive a PRVV

Bladder Care Assay

- Pangea Laboratory’s non-invasive Bladder CARE Assay has received BDD from the US FDA. It is quantitative urine-based diagnosis of bladder cancer or upper tract urothelial carcinoma (UTUC) in patients suffering with hematuria & above-mentioned cancer
- This assay measures the methylation levels of three biomarkers specific to UCC from single qPCR. The analysis outperformed traditional cytology and other FDA-approved tests, with specificities of 93.5% & 92.6%, 96.0% & 88.0% and 89.0% for the detection of bladder cancer, UTUC and sensitivity for carcinoma in-situ respectively
- Pangea laboratory is planning to start a multi-center trial to gain approval for Bladder CARE Assay
RadioGel Precision Radionuclide Therapy 
- Vivos’ RadioGel precision radionuclide therapy received BTD from the US FDA
- Animal study data verified Radiogel’s effectiveness & safety. Vivos & mayo clinic currently working together for use of RadioGel delivering therapeutic radiation to solid metastatic tumors associated with other indication
- RadioGel containsyttrium-90 phosphate microparticles which can directly administer into a tumor & localizes the dose within the treatment area by more than 90% of short-range beta radiation within 10 days so that tissues & normal organs are not adversely affected

OCU400

- Ocugen’s OCU400 received RMAT designation for retinitis pigmentosa (RP) associated with RHO mutations. It is based on preliminary clinical data of OCU400 -101 P-I/II study as measured by LLVA, MLMT & BCVA
- In the P-I/II study of OCU400, 86% (6/7) patients experienced either improvement or stabilization in MLMT scores from baseline, among which 29% (2/7) experienced 3 Lux luminance level improvement
- The data of gene-agnostic MOA of OCU400 suggest that it may be able to treat a group of RP & LCA patients. Ocugen’s intends to submit the safety & efficacy data to the FDA to expand its RMATD & for marketing authorization application and it is working with the FDA to decide the protocols necessary to advance the P-III study
4D-150

- 4DMT’s 4D-150 received RMATD from the US FDA for Intravitreal treatment of wet AMD. . RMAT designation follows PRIME designation received from the EMA in Oct 2023
- This RMATD is based on the interim results of P-I PRISM study which demonstrated tolerability, safety & clinical activity profile of 4D-150
- 4DMT is working on preliminary P-III study plan with the EMA & the US FDA & an update will be expected in Feb 2024 with interim randomized P-II PRISM clinical data from advanced, high treatment need wet AMD patients
Related Post: New Drug Designations – November 2023



