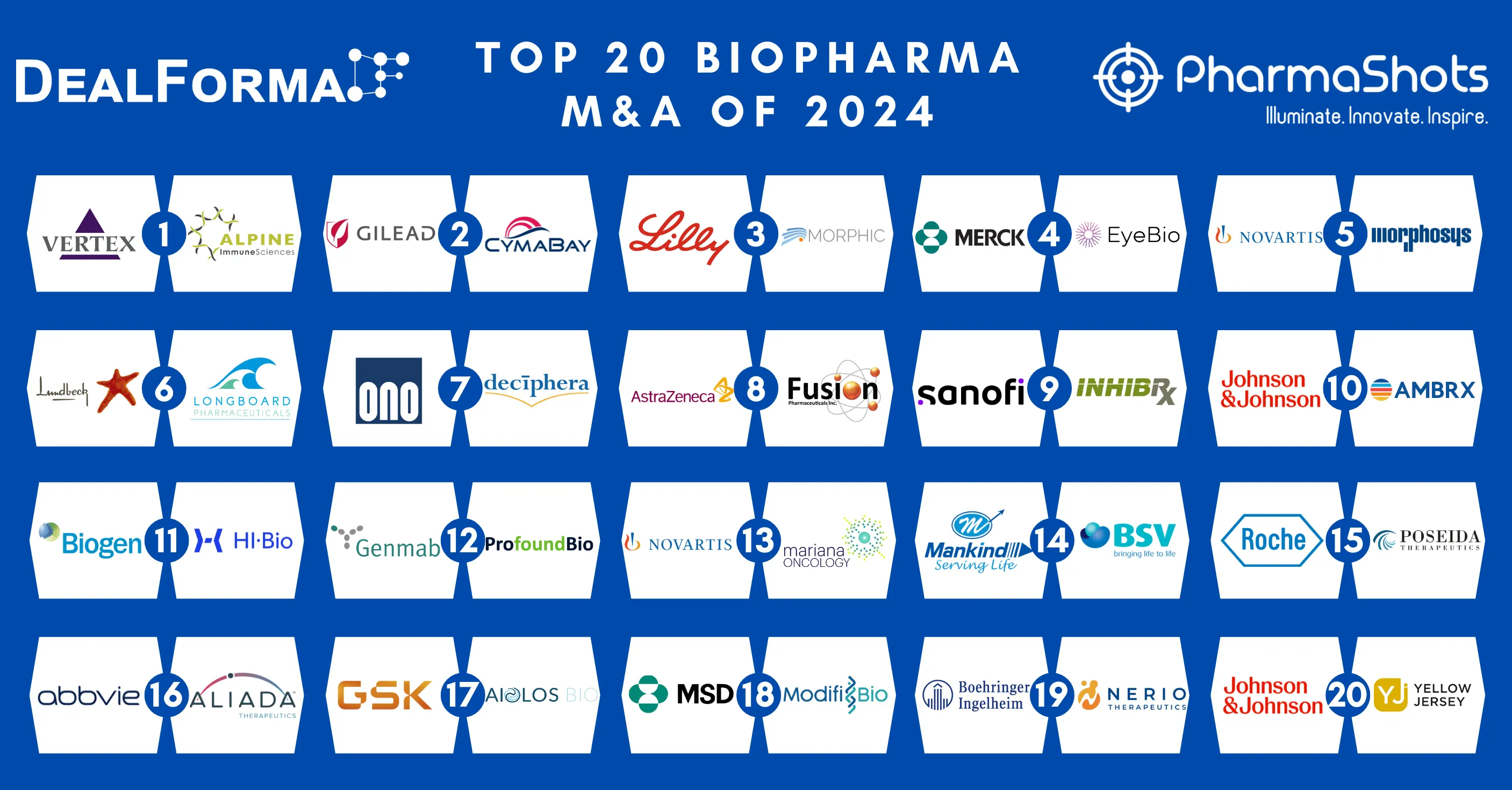
Fusion Pharmaceuticals Doses First Patient with FPI-2265 in P-II Study for Metastatic Castration-Resistant Prostate Cancer
Shots:
- Fusion has dosed the first patient with FPI-2265 under P-II part of the P-II/III (AlphaBreak) study for metastatic castration-resistant prostate cancer (mCRPC). The company anticipates the completion of patient recruitment (n=~60) in the P-II part by the end of 2024
- The P-II/III (AlphaBreak) study assesses the safety & efficacy of FPI-2265 in mCRPC patients who previously received 177Lu-PSMA radiotherapy. The P-II part aims at assessing 2 alternative dosing regimens' safety & efficacy vs previous dosing of 100 kBq/kg Q8W.
- The company further plans to enroll ~500 participants and the initiation of P-III global registration part of the study in 2025 post-determination of recommended P-III dosing regimen from the P-II part
Ref: AstraZeneca | Image: AstraZeneca
Related News:- AstraZeneca Acquires Fusion Pharmaceuticals for ~$2.4B to Development Radioconjugates for Cancer Treatment
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.