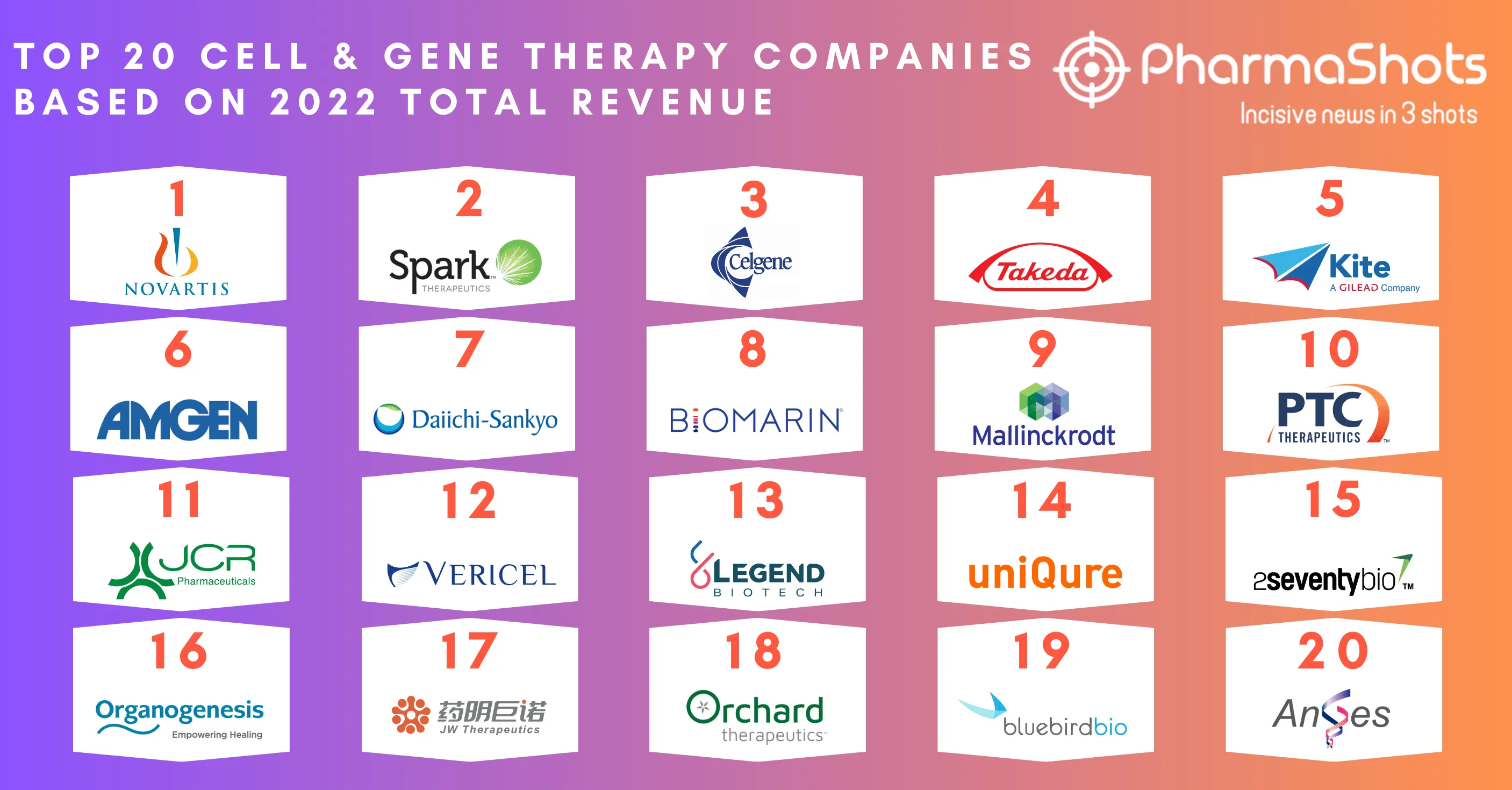
Organogenesis Reports the P-III Study Data of ReNu for Knee Osteoarthritis
Shots:
- Organogenesis reported data from the P-III study assessing the safety and efficacy of ReNu (2mL diluted with 2mL of saline or 4mL saline control, one intra-articular injection) vs saline control for managing symptoms among moderate to severe symptomatic knee OA patients (n=515)
- The study met its 1EPs, depicting knee pain reduction & maintained patient function after utilizing ReNu vs saline control at 6mos. with same AEs seen at 12mos. follow-up. Full analysis is anticipated in May. Another ReNu study is actively recruiting with full recruitment anticipated in 2024
- ReNu is a cryopreserved ASA containing amniotic fluid cells, micronized amniotic membrane, growth factors & extracellular matrix components
Ref: Organogenesis Inc. | Image: Organogenesis
Related News:- Organogenesis Reverse Merges with Avista Healthcare Public Acquisition Corp. (AHPAC)
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.