Disease of the Month: IgA Nephropathy
Shots:
- To create a wholesome environment for the current and the future generation, it’s totally up to life science companies and decision-makers to embrace and integrate every prospect of the underlying disease conditions and address those issues to find cures
- PharmaShots brings this month an informative take on the disease IgA nephropathy
- Often known as Berger’s disease, IgA nephropathy is a condition that occurs with the excess accumulation of an antibody called immunoglobulin A (IgA) in the kidneys
Introduction:
Deemed to be a rare disease, Immunoglobulin A (IgA) nephropathy AKA Berger’s disease is a condition that arises with the excess accumulation of Immunoglobulin A (IgA) antibody in glomeruli, leading kidneys to leak blood and protein into urine and local inflammation, affecting kidneys’ ability to filter waste from blood. A common form of glomerulonephritis, [1] IgA nephropathy may also be seen in hepatitis, cirrhosis, and HIV infection.
Though the prevalence of this disease is not constraint to a particular geographic location, the condition is mostly seen amongst Caucasian and Asian males.
IgA immune complexes are higher in circulation when patients have respiratory infections such as a cough or sore throat, therefore, many of them end up depositing in the kidneys and this is when patients with IgA nephropathy typically present with symptoms such as hematuria (blood in the urine).
Some patients may also experience IgA nephropathy in case of gastrointestinal infections incl. the stomach flu or even after exercise
Risk factors
- Gender: IgA nephropathy affects at least twice as many men as it does women
- Ethnicity: It is more common in whites and Asians than it is in blacks.
- Age: IgA nephropathy most often develops between the late teens and late 30s.
- Family history: It has been reported that IgA nephropathy is a complex polygenic disease that there are many genes and environmental factors that contribute to develop IgA nephropathy
Symptoms [2]
Signs and symptoms of IgA nephropathy include:
- Hematuria (caused by blood in the urine)
- Proteinuria
- Pain in the one or both sides of your back below your ribs
- Edema in hands and feet
- High blood pressure
Diagnosis: [3]
- Urine Tests: Doctor will collect urine for 24 hours for kidney function test to detect IgA nephropathy
- Blood Tests: In the presence of kidney disease, a blood test might show increased blood levels of the waste product creatinine
- Kidney Biopsy: The only way for doctors to confirm a diagnosis of IgA nephropathy. It involves using a special biopsy needle to extract kidney tissue for microscopic examination with light sedation and a local anesthetic The imaging techniques such as ultrasound or a CT scan is used to guide the biopsy needle into the kidney that show the IgA deposits in the glomeruli
- Iothalamate Clearance Test: It uses a special contrast agent to track filtration of wastes via kidneys
Epidemiology:[3]
A systematic study of biopsy-based literature reveals an incidence of over 2.5 per 100,000. IgA nephropathy is more common in Asian individuals (45 cases per million population/ year in Japan) than in Whites (31 cases per million population/ year in France)
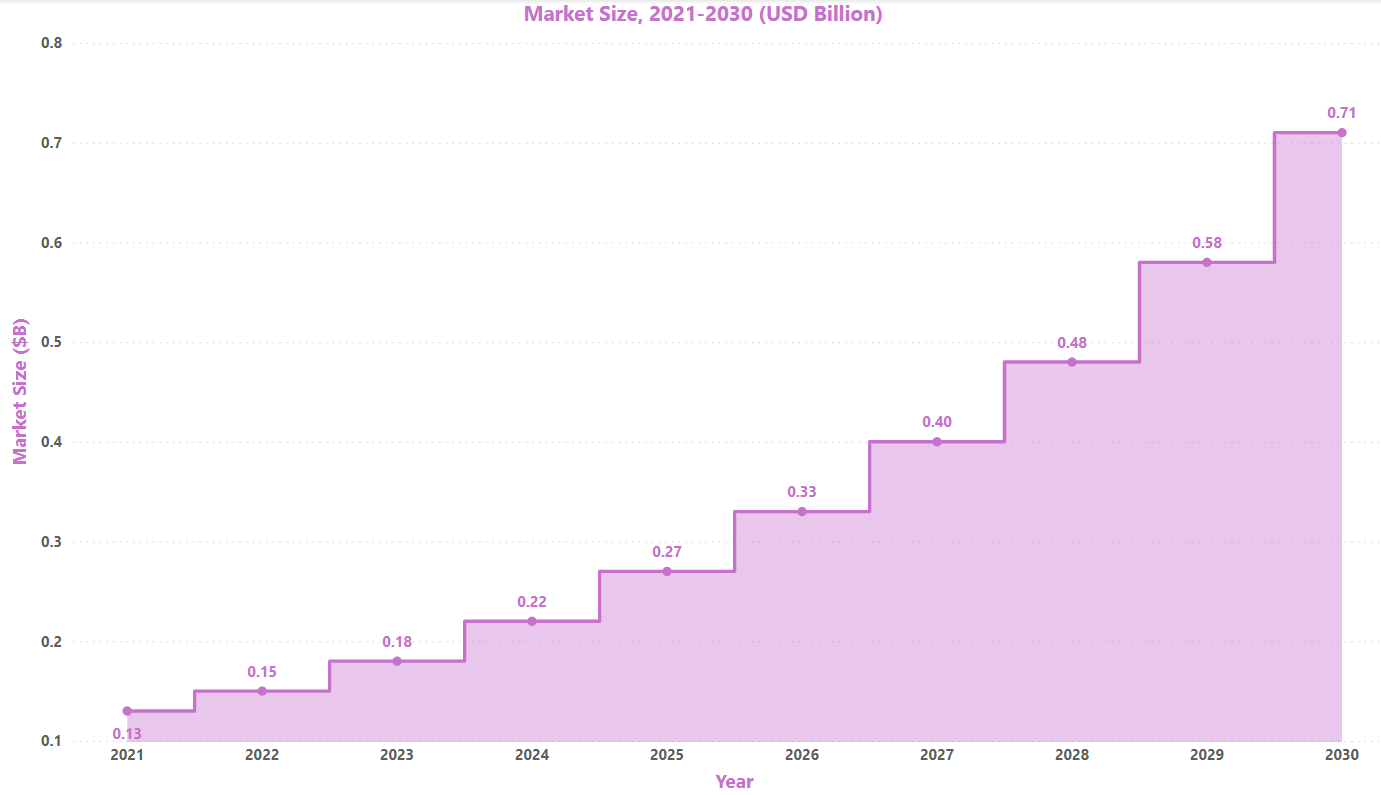
Market Size: [4]
According to research, the global IgA Nephropathy treatment market was worth $0.13B in 2021 and is expected to increase ~$0.71B by the year 2030 at a CAGR of 20.8%
Treatment:
- Corticosteroids/ Immunosuppressive drugs are used to stop immune system from attacking glomeruli
- Blood pressure medications (ACE inhibitors and ARBs) used to reduce protein loss and control blood pressure
- Reducing salt (sodium) and protein intake in food will lead to decrease the load of wastes on the kidneys
- Fish oil supplements
Key Players in the Market:
Filspari (sparsentan)[5]
- Sparsentan is developed by Travere Therapeutics
- In Feb 2023, Sparsentan received accelerated approval from the US FDA for the reduction of proteinuria in IgA Nephropathy patients
- It is a non-immunosuppressive therapy works as dual endothelin angiotensin receptor antagonist (DEARA) and targeting two pathways (endothelin-1 and angiotensin II)
- In H2’23, Travere Therapeutics along with its collaborator CSL Vi, expects EMA’s review decision on the Conditional Marketing Authorization of sparsentan for the treatment of IgAN in the EU
- It is available in 200 mg, oral, QD for 14 days, then 400 mg, QD as tolerated
- Budesonide is developed by Calliditas
- For China it is being developed in collaboration with Everest Medicines under the name NEFECON & in Europe, is being marketed by Stada Arzneimittel
- Calliditas formed a licensing agreement with Viatris to commercialize Nefecon in Japan
- It is a delayed release capsules used to reduce levels of protein in the urine (proteinuria) in adults with IgAN who are at high risk of disease progression
- In Dec 2021, Tarpeyo (budesonide) received accelerated approval from the US FDA and was subsequently launch in the US in Jan 2022 by Calliditas
- In Jul 2022, the EC granted approval to Kinpeygo (budesonide) for IgA nephropathy and later launched in Sep 2022 by Stada
Clinical Trial Analysis:[7]
Key players in this indication are – Chinook Therapeutics (BION 1301), RemeGen, Novartis (Iptacopan), Ionis Pharmaceuticals (IONIS-FB-LRx), Visterra (sibeprenlimab) etc.
Based on G10 geography distribution, the interventional clinical trials are classified in the below mentioned graph in two groups based on its status i.e., active (recruiting, active, not recruiting, not yet recruiting and enrolling by invitation) and inactive (withdrawn, suspended terminated and trials with unknow status). The interpretation showed that highest no. of trials being conducted in the USA and UK (17) and the least number of trials are reported in India (3)


*This graphical interpretation is based on our point of view of the clinical data
References:
1. Mayo Clinic
3. NIH
6. Tarpeyo
7. Kinpeygo
For Deep dive landscape please mail us at connect@pharmshots.com
Related Post:
1. https://www.pharmashots.com/14440/disease-of-the-month-age-related-macular-degeneration






